The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells
- PMID: 24412757
- PMCID: PMC4048963
- DOI: 10.1016/j.scr.2013.12.003
The negative impact of Wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells
Abstract
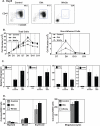
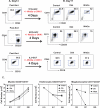
The Wnt gene family consists of structurally related genes encoding secreted signaling molecules that have been implicated in many developmental processes, including regulation of cell fate and patterning during embryogenesis. Previously, we found that Wnt signaling is required for primitive or yolk sac-derived-erythropoiesis using the murine embryonic stem cell (ESC) system. Here, we examine the effect of Wnt signaling on the formation of early hematopoietic progenitors derived from human ESCs. The first hematopoietic progenitor cells in the human ESC system express the pan-hematopoietic marker CD41 and the erythrocyte marker, glycophorin A or CD235. We have developed a novel serum-free, feeder-free, adherent differentiation system that can efficiently generate large numbers of CD41+CD235+ cells. We demonstrate that this cell population contains progenitors not just for primitive erythroid and megakaryocyte cells but for the myeloid lineage as well and term this population the primitive common myeloid progenitor (CMP). Treatment of mesoderm-specified cells with Wnt3a led to a loss of hematopoietic colony-forming ability while the inhibition of canonical Wnt signaling with DKK1 led to an increase in the number of primitive CMPs. Canonical Wnt signaling also inhibits the expansion and/or survival of primitive erythrocytes and megakaryocytes, but not myeloid cells, derived from this progenitor population. These findings are in contrast to the role of Wnt signaling during mouse ESC differentiation and demonstrate the importance of the human ESC system in studying species-specific differences in development.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Canonical Wnt signaling promotes early hematopoietic progenitor formation and erythroid specification during embryonic stem cell differentiation.PLoS One. 2013 Nov 26;8(11):e81030. doi: 10.1371/journal.pone.0081030. eCollection 2013. PLoS One. 2013. PMID: 24324557 Free PMC article.
-
MicroRNA screen of human embryonic stem cell differentiation reveals miR-105 as an enhancer of megakaryopoiesis from adult CD34+ cells.Stem Cells. 2014 May;32(5):1337-46. doi: 10.1002/stem.1640. Stem Cells. 2014. PMID: 24446170 Free PMC article.
-
The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis.Blood. 2007 Feb 15;109(4):1433-41. doi: 10.1182/blood-2006-06-031898. Epub 2006 Oct 24. Blood. 2007. PMID: 17062726 Free PMC article.
-
Hematopoietic stem cell-independent hematopoiesis: emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo.FEBS Lett. 2016 Nov;590(22):3965-3974. doi: 10.1002/1873-3468.12459. Epub 2016 Oct 27. FEBS Lett. 2016. PMID: 27790707 Review.
-
Erythro-myeloid progenitors: "definitive" hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells.Blood Cells Mol Dis. 2013 Dec;51(4):220-5. doi: 10.1016/j.bcmd.2013.09.006. Epub 2013 Oct 2. Blood Cells Mol Dis. 2013. PMID: 24095199 Free PMC article. Review.
Cited by
-
Identification and validation of ferroptosis related markers in erythrocyte differentiation of umbilical cord blood-derived CD34+ cell by bioinformatic analysis.Front Genet. 2024 Jul 30;15:1365232. doi: 10.3389/fgene.2024.1365232. eCollection 2024. Front Genet. 2024. PMID: 39139819 Free PMC article.
-
Arterial identity of hemogenic endothelium: a key to unlock definitive hematopoietic commitment in human pluripotent stem cell cultures.Exp Hematol. 2019 Mar;71:3-12. doi: 10.1016/j.exphem.2018.11.007. Epub 2018 Nov 28. Exp Hematol. 2019. PMID: 30500414 Free PMC article. Review.
-
GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells.Stem Cell Reports. 2017 Mar 14;8(3):589-604. doi: 10.1016/j.stemcr.2016.12.026. Epub 2017 Feb 9. Stem Cell Reports. 2017. PMID: 28196690 Free PMC article.
-
Downregulation of MIR100HG Induces Apoptosis in Human Megakaryoblastic Leukemia Cells.Indian J Hematol Blood Transfus. 2021 Apr;37(2):232-239. doi: 10.1007/s12288-020-01324-6. Epub 2020 Jul 24. Indian J Hematol Blood Transfus. 2021. PMID: 33867729 Free PMC article.
-
Utilization of the AAVS1 safe harbor locus for hematopoietic specific transgene expression and gene knockdown in human ES cells.Stem Cell Res. 2014 May;12(3):630-7. doi: 10.1016/j.scr.2014.02.004. Epub 2014 Feb 21. Stem Cell Res. 2014. PMID: 24631742 Free PMC article.
References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental biology. 2000;227(2):271–278. doi: 10.1006/dbio.2000.9912. - PubMed
-
- Chen AT, Zon LI. Zebrafish blood stem cells. Journal of cellular biochemistry. 2009;108(1):35–42. doi: 10.1002/jcb.22251. - PubMed
-
- Crispino JD. GATA1 in normal and malignant hematopoiesis. Seminars in cell & developmental biology. 2005;16(1):137–147. [Review] doi: 10.1016/j.semcdb.2004.11.002. - PubMed
-
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. Experimental hematology. 2005;33(9):955–964. [Review] doi: 10.1016/j.exphem.2005.06.009. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

