Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation
- PMID: 24412858
- PMCID: PMC3960325
- DOI: 10.1016/j.freeradbiomed.2014.01.001
Chronic ethanol ingestion induces oxidative kidney injury through taurine-inhibitable inflammation
Abstract
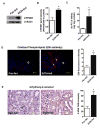
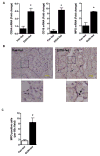
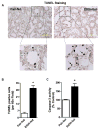
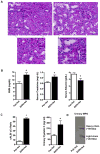
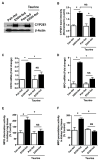
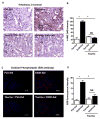
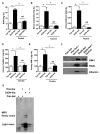
Chronic ethanol ingestion mildly damages liver through oxidative stress and lipid oxidation, which is ameliorated by dietary supplementation with the anti-inflammatory β-amino acid taurine. Kidney, like liver, expresses cytochrome P450 2E1 that catabolizes ethanol with free radical formation, and so also may be damaged by ethanol catabolism. Sudden loss of kidney function, and not liver disease itself, foreshadows mortality in patients with alcoholic hepatitis [J. Altamirano, Clin. Gastroenterol. Hepatol. 2012, 10:65]. We found that ethanol ingestion in the Lieber-deCarli rat model increased kidney lipid oxidation, 4-hydroxynonenal protein adduction, and oxidatively truncated phospholipids that attract and activate leukocytes. Chronic ethanol ingestion increased myeloperoxidase-expressing cells in kidney and induced an inflammatory cell infiltrate. Apoptotic terminal deoxynucleotidyl transferase nick-end labeling-positive cells and active caspase-3 increased in kidney after ethanol ingestion, with reduced filtration with increased circulating blood urea nitrogen (BUN) and creatinine. These events were accompanied by release of albumin, myeloperoxidase, and the acute kidney injury biomarkers kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin, and cystatin c into urine. Taurine sequesters HOCl from myeloperoxidase of activated leukocytes, and taurine supplementation reduced renal lipid oxidation, reduced leukocyte infiltration, and reduced the increase in myeloperoxidase-positive cells during ethanol feeding. Taurine supplementation also normalized circulating BUN and creatinine levels and suppressed enhanced myeloperoxidase, albumin, KIM-1, and cystatin c in urine. Thus, chronic ethanol ingestion oxidatively damages kidney lipids and proteins, damages renal function, and induces acute kidney injury through an inflammatory cell infiltrate. The anti-inflammatory nutraceutical taurine effectively interrupts this ethanol-induced inflammatory cycle in kidney.
Keywords: Acute kidney injury; Free radicals; Inflammation; Kidney; Oxidized phospholipid; Reactive oxygen species; Taurine.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. - PubMed
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. New Engl’d J Med. 2009;360:2758–2769. - PubMed
-
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. - PubMed
-
- Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

