Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo
- PMID: 24412922
- PMCID: PMC3966466
- DOI: 10.1038/nm.3443
Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo
Abstract
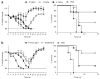
Neutralizing antibodies against influenza viruses have traditionally been thought to provide protection exclusively through their variable region; the contributions of mechanisms conferred by the Fc domain remain controversial. We investigated the in vivo contributions of Fc interactions with their cognate receptors for a collection of neutralizing anti-influenza antibodies. Whereas five broadly neutralizing monoclonal antibodies (bNAbs) targeting the conserved stalk region of hemagglutinin (HA) required interactions between the antibody Fc and Fc receptors for IgG (FcγRs) to confer protection from lethal H1N1 challenge, three strain-specific monoclonal Abs (mAbs) against the variable head domain of HA were equally protective in the presence or absence of FcγR interactions. Although all antibodies blocked infection, only anti-stalk bNAbs were capable of mediating cytotoxicity of infected cells, which accounts for their FcγR dependence. Immune complexes generated with anti-HA stalk mAb efficiently interacted with FcγRs, but anti-HA head immune complexes did not. These results suggest that FcγR binding capacity by anti-HA antibodies was dependent on the interaction of the cognate Fab with antigen. We exploited these disparate mechanisms of mAb-mediated protection to reengineer an anti-stalk bNAb to selectively enhance FcγR engagement to augment its protective activity. These findings reveal a previously uncharacterized property of bNAbs and guide an approach toward enhancing mAb-mediated antiviral therapeutics.
Figures
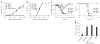

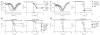
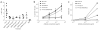
References
-
- World Health Organization Influenza (Seasonal) Fact sheet. 2009:211. http://www.who.int/mediacentre/factsheets/fs211/en/
-
- Martinez O, Tsibane T, Basler CF. Neutralizing anti-influenza virus monoclonal antibodies: therapeutics and tools for discovery. Int. Rev. Immunol. 2009;28:69–92. - PubMed
-
- Couch RB, Kasel JA. Immunity to influenza in man. Annu. Rev. Microbiol. 1983;37:529–549. - PubMed
-
- Wang TT, Palese P. Biochemistry. Catching a moving target. Science. 2011;333:834–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

