Mutation of the Arabidopsis LON2 peroxisomal protease enhances pexophagy
- PMID: 24413187
- PMCID: PMC4077889
- DOI: 10.4161/auto.27565
Mutation of the Arabidopsis LON2 peroxisomal protease enhances pexophagy
Abstract
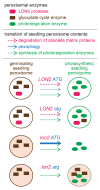
Peroxisomes are critical organelles housing various, often oxidative, reactions. Pexophagy, the process by which peroxisomes are selectively targeted for destruction via autophagy, is characterized in yeast and mammals but had not been reported in plants. In this article, we describe how the peroxisome-related aberrations of a mutant defective in the LON2 peroxisomal protease are suppressed when autophagy is prevented by mutating any of several key autophagy-related (ATG) genes. Our results reveal that plant peroxisomes can be degraded by selective autophagy and suggest that pexophagy is accelerated when the LON2 protease is disabled.
Keywords: Arabidopsis thaliana; autophagy; peroxisomal protease; peroxisome remodeling; pexophagy.
Figures
Comment on
- Farmer LM, Rinaldi MA, Young PG, Danan CH, Burkhart SE, Bartel B. Disrupting autophagy restores peroxisome function to an Arabidopsis lon2 mutant and reveals a role for the LON2 protease in peroxisomal matrix protein degradation. Plant Cell. 2013;25:4085–100. doi: 10.1105/tpc.113.113407. doi: 10.1105/tpc.113.113407
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
