Heteropathogenic virulence and phylogeny reveal phased pathogenic metamorphosis in Escherichia coli O2:H6
- PMID: 24413188
- PMCID: PMC3958309
- DOI: 10.1002/emmm.201303133
Heteropathogenic virulence and phylogeny reveal phased pathogenic metamorphosis in Escherichia coli O2:H6
Abstract
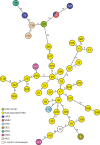
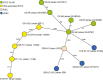
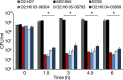
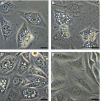
Extraintestinal pathogenic and intestinal pathogenic (diarrheagenic) Escherichia coli differ phylogenetically and by virulence profiles. Classic theory teaches simple linear descent in this species, where non-pathogens acquire virulence traits and emerge as pathogens. However, diarrheagenic Shiga toxin-producing E. coli (STEC) O2:H6 not only possess and express virulence factors associated with diarrheagenic and uropathogenic E. coli but also cause diarrhea and urinary tract infections. These organisms are phylogenetically positioned between members of an intestinal pathogenic group (STEC) and extraintestinal pathogenic E. coli. STEC O2:H6 is, therefore, a 'heteropathogen,' and the first such hybrid virulent E. coli identified. The phylogeny of these E. coli and the repertoire of virulence traits they possess compel consideration of an alternate view of pathogen emergence, whereby one pathogroup of E. coli undergoes phased metamorphosis into another. By understanding the evolutionary mechanisms of bacterial pathogens, rational strategies for counteracting their detrimental effects on humans can be developed.
Figures


References
-
- Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–1248. - PubMed
-
- Bielaszewska M, Friedrich AW, Aldick T, Schurk-Bulgrin R, Karch H. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin Infect Dis. 2006;43:1160–1167. - PubMed
-
- Bielaszewska M, Mellmann A, Bletz S, Zhang W, Kock R, Kossow A, Prager R, Fruth A, Orth-Holler D, Marejkova M, et al. Enterohemorrhagic Escherichia coli O26:H11/H−: a new virulent clone emerges in Europe. Clin Infect Dis. 2013;56:1373–1381. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- SRA/PRJEB4756
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

