Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells
- PMID: 24413384
- PMCID: PMC4004082
- DOI: 10.1093/molehr/gau001
Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells
Abstract
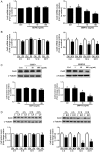
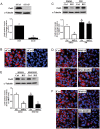
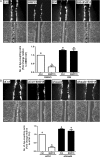
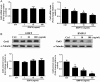
In the ovary, connexin-coupled gap junctions in granulosa cells play crucial roles in follicular and oocyte development as well as in corpus luteum formation. Our previous work has shown that theca cell-derived bone morphogenetic protein (BMP)4 and BMP7 decrease gap junction intercellular communication (GJIC) activity via the down-regulation of connexin43 (Cx43) expression in immortalized human granulosa cells. However, the effects of oocyte-derived growth factors on Cx43 expression remain to be elucidated. The present study was designed to investigate the effects of oocyte-derived growth differentiation factor (GDF)9 and BMP15 on the expression of Cx43 in a human granulosa cell line, SVOG. We also examined the effect relative to GJIC activity and investigated the potential mechanisms of action. In SVOG cells, treatment with BMP15 but not GDF9 significantly decreased Cx43 mRNA and protein levels and GJIC activity. These suppressive effects, along with the induction of Smad1/5/8 phosphorylation, were attenuated by co-treatment with a BMP type I receptor inhibitor, dorsomorphin. Furthermore, knockdown of the central component of the transforming growth factor-β superfamily signaling pathway, Smad4, using small interfering RNA reversed the suppressive effects of BMP15 on Cx43 expression and GJIC activity. The suppressive effects of BMP15 on Cx43 expression were further confirmed in primary human granulosa-lutein cells obtained from infertile patients undergoing an in vitro fertilization procedure. These findings suggest that oocyte-derived BMP15 decreases GJIC activity between human granulosa cells by down-regulating Cx43 expression, most likely via a Smad-dependent signaling pathway.
Keywords: BMP15; GDF9; connexin43; human granulosa cell; smad.
Figures


Similar articles
-
Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells.J Clin Endocrinol Metab. 2013 Mar;98(3):E437-45. doi: 10.1210/jc.2012-3851. Epub 2013 Feb 5. J Clin Endocrinol Metab. 2013. PMID: 23386650
-
Bone morphogenetic protein 2 regulates cell-cell communication by down-regulating connexin43 expression in luteinized human granulosa cells.Mol Hum Reprod. 2017 Mar 1;23(3):155-165. doi: 10.1093/molehr/gaw078. Mol Hum Reprod. 2017. PMID: 27986931
-
BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells.Mol Endocrinol. 2013 Dec;27(12):2093-104. doi: 10.1210/me.2013-1233. Epub 2013 Oct 18. Mol Endocrinol. 2013. PMID: 24140593 Free PMC article.
-
The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis.Reprod Domest Anim. 2011 Apr;46(2):354-61. doi: 10.1111/j.1439-0531.2010.01739.x. Epub 2010 Dec 30. Reprod Domest Anim. 2011. PMID: 21198974 Review.
-
The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders.Hum Reprod Update. 2014 Nov-Dec;20(6):869-83. doi: 10.1093/humupd/dmu036. Epub 2014 Jun 30. Hum Reprod Update. 2014. PMID: 24980253 Review.
Cited by
-
Morphokinetic analysis of cleavage stage embryos and assessment of specific gene expression in cumulus cells independently predict human embryo development to expanded blastocyst: a preliminary study.J Assist Reprod Genet. 2020 Jun;37(6):1409-1420. doi: 10.1007/s10815-020-01806-6. Epub 2020 May 20. J Assist Reprod Genet. 2020. PMID: 32436046 Free PMC article.
-
GDF-9 and BMP-15 direct the follicle symphony.J Assist Reprod Genet. 2018 Oct;35(10):1741-1750. doi: 10.1007/s10815-018-1268-4. Epub 2018 Jul 23. J Assist Reprod Genet. 2018. PMID: 30039232 Free PMC article. Review.
-
Response Surface Methodology for Statistical Optimization of Chitosan/Alginate Nanoparticles as a Vehicle for Recombinant Human Bone Morphogenetic Protein-2 Delivery.Int J Nanomedicine. 2020 Oct 29;15:8345-8356. doi: 10.2147/IJN.S250630. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33154637 Free PMC article.
-
Influence of follicular fluid and cumulus cells on oocyte quality: clinical implications.J Assist Reprod Genet. 2018 May;35(5):735-751. doi: 10.1007/s10815-018-1143-3. Epub 2018 Mar 2. J Assist Reprod Genet. 2018. PMID: 29497954 Free PMC article. Review.
-
Bisphenol S enhances gap junction intercellular communication in ovarian theca cells.Chemosphere. 2021 Jan;263:128304. doi: 10.1016/j.chemosphere.2020.128304. Epub 2020 Sep 14. Chemosphere. 2021. PMID: 33155548 Free PMC article.
References
-
- Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. - PubMed
-
- Andersen J, Grine E, Eng CL, Zhao K, Barbieri RL, Chumas JC, Brink PR. Expression of connexin-43 in human myometrium and leiomyoma. Am J Obstet Gynecol. 1993;169:1266–1276. - PubMed
-
- Beyer EC, Paul DL, Goodenough DA. Connexin family of gap junction proteins. J Membr Biol. 1990;116:187–194. - PubMed
-
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

