Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells
- PMID: 24413397
- PMCID: PMC4030630
- DOI: 10.1038/nature12923
Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells
Abstract
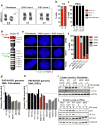
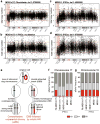
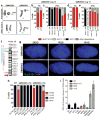
Ring chromosomes are structural aberrations commonly associated with birth defects, mental disabilities and growth retardation. Rings form after fusion of the long and short arms of a chromosome, and are sometimes associated with large terminal deletions. Owing to the severity of these large aberrations that can affect multiple contiguous genes, no possible therapeutic strategies for ring chromosome disorders have been proposed. During cell division, ring chromosomes can exhibit unstable behaviour leading to continuous production of aneuploid progeny with low viability and high cellular death rate. The overall consequences of this chromosomal instability have been largely unexplored in experimental model systems. Here we generated human induced pluripotent stem cells (iPSCs) from patient fibroblasts containing ring chromosomes with large deletions and found that reprogrammed cells lost the abnormal chromosome and duplicated the wild-type homologue through the compensatory uniparental disomy (UPD) mechanism. The karyotypically normal iPSCs with isodisomy for the corrected chromosome outgrew co-existing aneuploid populations, enabling rapid and efficient isolation of patient-derived iPSCs devoid of the original chromosomal aberration. Our results suggest a fundamentally different function for cellular reprogramming as a means of 'chromosome therapy' to reverse combined loss-of-function across many genes in cells with large-scale aberrations involving ring structures. In addition, our work provides an experimentally tractable human cellular system for studying mechanisms of chromosomal number control, which is of critical relevance to human development and disease.
Figures

Comment in
-
Genome stability: Chromosome correction through reprogramming.Nat Rev Genet. 2014 Mar;15(3):146. doi: 10.1038/nrg3680. Epub 2014 Feb 4. Nat Rev Genet. 2014. PMID: 24492236 No abstract available.
References
Publication types
MeSH terms
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

