Patient subgroup analyses of the treatment effect of subcutaneous interferon β-1a on development of multiple sclerosis in the randomized controlled REFLEX study
- PMID: 24413638
- PMCID: PMC3948518
- DOI: 10.1007/s00415-013-7222-6
Patient subgroup analyses of the treatment effect of subcutaneous interferon β-1a on development of multiple sclerosis in the randomized controlled REFLEX study
Abstract
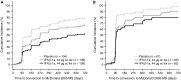
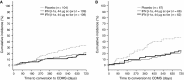
The REFLEX study (NCT00404352) established that subcutaneous (sc) interferon (IFN) β-1a reduced the risks of McDonald MS (2005 criteria) and clinically definite multiple sclerosis (CDMS) in patients with a first clinical demyelinating event suggestive of MS. The aim of this subgroup analysis was to assess the treatment effect of sc IFN β-1a in patient subgroups defined by baseline disease and demographic characteristics (age, sex, use of steroids at the first event, classification of first event as mono- or multifocal, presence/absence of gadolinium-enhancing lesions, count of <9 or ≥9 T2 lesions), and by diagnosis of MS using the revised McDonald 2010 MS criteria. Patients were randomized to the serum-free formulation of IFN β-1a, 44 μg sc three times weekly or once weekly, or placebo, for 24 months or until diagnosis of CDMS. Treatment effects of sc IFN β-1a on McDonald 2005 MS and CDMS in the predefined subgroups were similar to effects found in the intent-to-treat population. McDonald 2010 MS was retrospectively diagnosed in 37.7 % of patients at baseline. Both regimens of sc IFN β-1a significantly reduced the risk versus placebo of McDonald 2005 MS and CDMS, irrespective of McDonald 2010 status at baseline (risk reductions between 29 and 51 %). The effect of sc IFN β-1a was not substantially influenced by baseline patient demographic and disease characteristics, or baseline presence/absence of McDonald 2010 MS.
Figures




References
-
- Brex PA, Miszkiel KA, O’Riordan JI, Plant GT, Moseley IF, Thompson AJ, Miller DH. Assessing the risk of early multiple sclerosis in patients with clinically isolated syndromes: the role of a follow up MRI. J Neurol Neurosurg Psychiatry. 2001;70:390–393. doi: 10.1136/jnnp.70.3.390. - DOI - PMC - PubMed
-
- Tintoré M, Rovira A, Rio J, Nos C, Grivé E, Téllez N, Pelayo R, Comabella M, Sastre-Garriga J, Montalban X. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006;67:968–972. doi: 10.1212/01.wnl.0000237354.10144.ec. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

