Inhibition of Tau aggregation by three Aspergillus nidulans secondary metabolites: 2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde
- PMID: 24414310
- PMCID: PMC6442474
- DOI: 10.1055/s-0033-1360180
Inhibition of Tau aggregation by three Aspergillus nidulans secondary metabolites: 2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde
Abstract
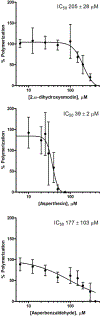
The aggregation of the microtubule-associated protein tau is a significant event in many neurodegenerative diseases including Alzheimer's disease. The inhibition or reversal of tau aggregation is therefore a potential therapeutic strategy for these diseases. Fungal natural products have proven to be a rich source of useful compounds having wide varieties of biological activity. We have screened Aspergillus nidulans secondary metabolites containing aromatic ring structures for their ability to inhibit tau aggregation in vitro using an arachidonic acid polymerization protocol and the previously identified aggregation inhibitor emodin as a positive control. While several compounds showed some activity, 2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde were potent aggregation inhibitors as determined by both a filter trap assay and electron microscopy. In this study, these three compounds were stronger inhibitors than emodin, which has been shown in a prior study to inhibit the heparin induction of tau aggregation with an IC50 of 1-5 µM. Additionally, 2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde reduced, but did not block, tau stabilization of microtubules. 2,ω-Dihydroxyemodin and asperthecin have similar structures to previously identified tau aggregation inhibitors, while asperbenzaldehyde represents a new class of compounds with tau aggregation inhibitor activity. Asperbenzaldehyde can be readily modified into compounds with strong lipoxygenase inhibitor activity, suggesting that compounds derived from asperbenzaldehyde could have dual activity. Together, our data demonstrates the potential of 2,ω-dihydroxyemodin, asperthecin, and asperbenzaldehyde as lead compounds for further development as therapeutics to inhibit tau aggregation in Alzheimer's disease and neurodegenerative tauopathies.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures




Similar articles
-
Fungally Derived Isoquinoline Demonstrates Inducer-Specific Tau Aggregation Inhibition.Biochemistry. 2021 Jun 1;60(21):1658-1669. doi: 10.1021/acs.biochem.1c00111. Epub 2021 May 19. Biochemistry. 2021. PMID: 34009955 Free PMC article.
-
Azaphilones inhibit tau aggregation and dissolve tau aggregates in vitro.ACS Chem Neurosci. 2015 May 20;6(5):751-60. doi: 10.1021/acschemneuro.5b00013. Epub 2015 Apr 15. ACS Chem Neurosci. 2015. PMID: 25822288 Free PMC article.
-
Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells.J Biol Chem. 2005 Feb 4;280(5):3628-35. doi: 10.1074/jbc.M410984200. Epub 2004 Nov 2. J Biol Chem. 2005. PMID: 15525637
-
Tau as a therapeutic target in neurodegenerative disease.Pharmacol Ther. 2012 Oct;136(1):8-22. doi: 10.1016/j.pharmthera.2012.07.001. Epub 2012 Jul 10. Pharmacol Ther. 2012. PMID: 22790092 Free PMC article. Review.
-
Development of tau aggregation inhibitors for Alzheimer's disease.Angew Chem Int Ed Engl. 2009;48(10):1740-52. doi: 10.1002/anie.200802621. Angew Chem Int Ed Engl. 2009. PMID: 19189357 Review.
Cited by
-
Polyketides as Secondary Metabolites from the Genus Aspergillus.J Fungi (Basel). 2023 Feb 15;9(2):261. doi: 10.3390/jof9020261. J Fungi (Basel). 2023. PMID: 36836375 Free PMC article. Review.
-
EGCG impedes human Tau aggregation and interacts with Tau.Sci Rep. 2020 Jul 28;10(1):12579. doi: 10.1038/s41598-020-69429-6. Sci Rep. 2020. PMID: 32724104 Free PMC article.
-
Fungally Derived Isoquinoline Demonstrates Inducer-Specific Tau Aggregation Inhibition.Biochemistry. 2021 Jun 1;60(21):1658-1669. doi: 10.1021/acs.biochem.1c00111. Epub 2021 May 19. Biochemistry. 2021. PMID: 34009955 Free PMC article.
-
Conversion of Polyethylenes into Fungal Secondary Metabolites.Angew Chem Int Ed Engl. 2023 Jan 23;62(4):e202214609. doi: 10.1002/anie.202214609. Epub 2022 Nov 23. Angew Chem Int Ed Engl. 2023. PMID: 36417558 Free PMC article.
-
Neem Derivatives Inhibits Tau Aggregation.J Alzheimers Dis Rep. 2019 Jun 14;3(1):169-178. doi: 10.3233/ADR-190118. J Alzheimers Dis Rep. 2019. PMID: 31259310 Free PMC article.
References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992; 42: 631–639 - PubMed
-
- Goedert M, Crowther RA, Spillantini MG Tau mutations cause frontotemporal dementias. Neuron 1998; 21: 955–958 - PubMed
-
- Goedert M, Ghetti B, Spillantini MG. Tau gene mutations in frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Their relevance for understanding the neurogenerative process. Ann N Y Acad Sci 2000; 920: 74–83 - PubMed
-
- Ko LW, DeTure M, Sahara N, Chihab R Vega IE, Yen SH. Recent advances in experimental modeling of the assembly of tau filaments. Biochim Biophys Acta 2005; 1739: 125–139 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

