Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma
- PMID: 24414535
- PMCID: PMC4022215
- DOI: 10.1093/neuonc/not241
Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma
Abstract
Background: The purpose of this study was to evaluate amifostine for protection from cisplatin-induced serious hearing loss in patients with average-risk medulloblastoma by extending a previous analysis to a much larger sample size. In addition, this study aimed to assess amifostine with serious hearing loss in patients with high-risk medulloblastoma treated with cisplatin.
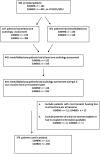
Methods: Newly diagnosed medulloblastoma patients (n = 379; ages 3-21 years), enrolled on one of 2 sequential St. Jude clinical protocols that included 4 courses of 75 mg/m(2) cisplatin, were compared for hearing loss by whether or not they received 600 mg/m(2) of amifostine immediately before and 3 hours into each cisplatin infusion. Amifostine administration was not randomized. The last audiological evaluation between 5.5 and 24.5 months following protocol treatment initiation was graded using the Chang Ototoxicity Scale. A grade of ≥ 2b (loss requiring a hearing aid or deafness) was considered a serious event.
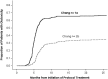
Results: Among average-risk patients (n = 263), amifostine was associated with protection from serious hearing loss (adjusted OR, 0.30; 95% CI, 0.14-0.64). For high-risk patients (n = 116), however, there was not sufficient evidence to conclude that amifostine prevented serious hearing loss (OR, 0.89; 95% CI, 0.31-2.54).
Conclusions: Although patients in this study were not randomly assigned to amifostine treatment, we found evidence in favor of amifostine administration for protection against cisplatin-induced serious hearing loss in average-risk but not in high-risk, medulloblastoma patients.
Keywords: audiology; brain neoplasms; late effects; ototoxicity; platinum drugs.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Orgel E, Jain S, Ji L, et al. Hearing loss among survivors of childhood brain tumors treated with an irradiation-sparing approach. Pediatr Blood Cancer. 2012;58:953–958. - PubMed
-
- Lafay-Cousin L, Purdy E, Huang A, et al. Early cisplatin induced ototoxicity profile may predict the need for hearing support in children with medulloblastoma. Pediatr Blood Cancer. 2013;60:287–292. - PubMed
-
- Van Der Hulst RJAM, Dreschler WA, Urbanus NAM. High frequency audiometry in prospective clinical research of ototoxicity due to platinum derivatives. Ann Otol Rhinol and Laryngol. 1998;97:133–137. - PubMed
-
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources