mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma
- PMID: 24414536
- PMCID: PMC3956353
- DOI: 10.1093/neuonc/not242
mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma
Abstract
Background: Neurofibromatosis type 2 (NF2) is a rare autosomal dominant genetic disorder, resulting in a variety of neural tumors, with bilateral vestibular schwannomas as the most frequent manifestation. Recently, merlin, the NF2 tumor suppressor, has been identified as a novel negative regulator of mammalian target of rapamycin complex 1 (mTORC1); functional loss of merlin was shown to result in elevated mTORC1 signaling in NF2-related tumors. Thus, mTORC1 pathway inhibition may be a useful targeted therapeutic approach.
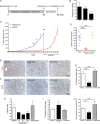
Methods: We studied in vitro cell models, cohorts of mice allografted with Nf2(-/-) Schwann cells, and a genetically modified mouse model of NF2 schwannoma in order to evaluate the efficacy of the proposed targeted therapy for NF2.
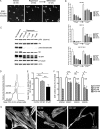
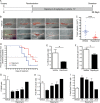
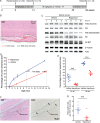
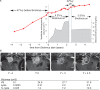
Results: We found that treatment with the mTORC1 inhibitor rapamycin reduced the severity of NF2-related Schwann cell tumorigenesis without significant toxicity. Consistent with these results, in an NF2 patient with growing vestibular schwannomas, the rapalog sirolimus induced tumor growth arrest.
Conclusions: Taken together, these results constitute definitive evidence that justifies proceeding with clinical trials using mTORC1-targeted agents in selected patients with NF2 and in patients with NF2-related sporadic tumors.
Keywords: neurofibromatosis type 2; rapamycin; schwannoma.
Figures
Comment in
-
Chemoprevention for neurofibromatosis 2: just over the horizon?Neuro Oncol. 2014 Apr;16(4):471-2. doi: 10.1093/neuonc/nou037. Neuro Oncol. 2014. PMID: 24637549 Free PMC article. No abstract available.
References
-
- Evans DG, Huson SM, Donnai D, et al. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603–618. - PubMed
-
- Hadfield KD, Smith MJ, Urquhart JE, et al. Rates of loss of heterozygosity and mitotic recombination in NF2 schwannomas, sporadic vestibular schwannomas and schwannomatosis schwannomas. Oncogene. 2010;29:6216–6221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

