Endothelial cells expressing low levels of CD143 (ACE) exhibit enhanced sprouting and potency in relieving tissue ischemia
- PMID: 24414940
- PMCID: PMC4063867
- DOI: 10.1007/s10456-014-9414-9
Endothelial cells expressing low levels of CD143 (ACE) exhibit enhanced sprouting and potency in relieving tissue ischemia
Abstract
The sprouting of endothelial cells from pre-existing blood vessels represents a critical event in the angiogenesis cascade. However, only a fraction of cultured or transplanted endothelial cells form new vessels. Moreover, it is unclear whether this results from a stochastic process or instead relates to certain endothelial cells having a greater angiogenic potential. This study investigated whether there exists a sub-population of cultured endothelial cells with enhanced angiogenic potency in vitro and in vivo. First, endothelial cells that participated in sprouting, and non-sprouting cells, were separately isolated from a 3D fibrin gel sprouting assay. Interestingly, the sprouting cells, when placed back into the same assay, displayed a sevenfold increase in the number of sprouts, as compared to control cells. Angiotensin-converting enzyme (CD143) was significantly down regulated on sprouting cells, as compared to regular endothelial cells. A subset of endothelial cells with low CD143 expression was then prospectively isolated from an endothelial cell culture. Finally, these cells were found to have greater potency in alleviating local ischemia, and restoring regional blood perfusion when transplanted into ischemic hindlimbs, as compared to unsorted endothelial cells. In summary, this study indicates that low expression of CD143 can be used as a biomarker to identify an endothelial cell sub-population that is more capable to drive neovascularization.
Figures
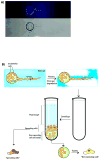
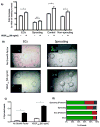


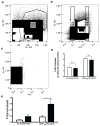
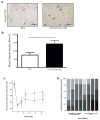
 ), one necrotic toe (
), one necrotic toe (
 ), two or more necrotic toes (
), two or more necrotic toes (
 ), necrotic foot (
), necrotic foot (
 ) and autoamputation (■). Mean values are presented with standard deviations, * indicates statistically significant differences (p<0.05) between conditions. Arrows in (A) highlight positive CD31 cells.
) and autoamputation (■). Mean values are presented with standard deviations, * indicates statistically significant differences (p<0.05) between conditions. Arrows in (A) highlight positive CD31 cells.Similar articles
-
WKYMVm-induced activation of formyl peptide receptor 2 stimulates ischemic neovasculogenesis by promoting homing of endothelial colony-forming cells.Stem Cells. 2014 Mar;32(3):779-90. doi: 10.1002/stem.1578. Stem Cells. 2014. PMID: 24155208
-
Injury-Mediated Vascular Regeneration Requires Endothelial ER71/ETV2.Arterioscler Thromb Vasc Biol. 2016 Jan;36(1):86-96. doi: 10.1161/ATVBAHA.115.306430. Epub 2015 Nov 19. Arterioscler Thromb Vasc Biol. 2016. PMID: 26586661 Free PMC article.
-
A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization.Acta Biomater. 2016 May;36:86-98. doi: 10.1016/j.actbio.2016.03.002. Epub 2016 Mar 4. Acta Biomater. 2016. PMID: 26961805 Free PMC article.
-
Role of endothelial microRNA-23 clusters in angiogenesis in vivo.Am J Physiol Heart Circ Physiol. 2018 Oct 1;315(4):H838-H846. doi: 10.1152/ajpheart.00742.2017. Epub 2018 Jun 15. Am J Physiol Heart Circ Physiol. 2018. PMID: 29906231
-
Dynamics of endothelial cell behavior in sprouting angiogenesis.Curr Opin Cell Biol. 2010 Oct;22(5):617-25. doi: 10.1016/j.ceb.2010.08.010. Curr Opin Cell Biol. 2010. PMID: 20817428 Review.
Cited by
-
Enzymatically degradable alginate hydrogel systems to deliver endothelial progenitor cells for potential revasculature applications.Biomaterials. 2018 Oct;179:109-121. doi: 10.1016/j.biomaterials.2018.06.038. Epub 2018 Jun 27. Biomaterials. 2018. PMID: 29980073 Free PMC article.
-
Up-Regulation of Angiotensin-Converting Enzyme (ACE) Enhances Cell Proliferation and Predicts Poor Prognosis in Laryngeal Cancer.Med Sci Monit. 2016 Nov 1;22:4132-4138. doi: 10.12659/msm.896933. Med Sci Monit. 2016. PMID: 27801393 Free PMC article.
-
Contribution of Human Fibroblasts and Endothelial Cells to the Hallmarks of Inflammation as Determined by Proteome Profiling.Mol Cell Proteomics. 2016 Jun;15(6):1982-97. doi: 10.1074/mcp.M116.058099. Epub 2016 Mar 29. Mol Cell Proteomics. 2016. PMID: 27025457 Free PMC article.
-
High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources.Stem Cell Res Ther. 2018 Jan 16;9(1):10. doi: 10.1186/s13287-017-0755-3. Stem Cell Res Ther. 2018. PMID: 29338788 Free PMC article.
-
Direct Vascular Effects of Angiotensin II (A Systematic Short Review).Int J Mol Sci. 2024 Dec 26;26(1):113. doi: 10.3390/ijms26010113. Int J Mol Sci. 2024. PMID: 39795971 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

