Omalizumab for asthma in adults and children
- PMID: 24414989
- PMCID: PMC10981784
- DOI: 10.1002/14651858.CD003559.pub4
Omalizumab for asthma in adults and children
Abstract
Background: Asthma is a respiratory (airway) condition that affects an estimated 300 million people worldwide and is associated with significant morbidity and mortality. Omalizumab is a monoclonal antibody that binds and inhibits free serum immunoglobulin E (IgE). It is called an 'anti-IgE' drug. IgE is an immune mediator involved in clinical manifestations of asthma. A recent update of National Institute for Health and Care Excellence (NICE) guidance in 2013 recommends omalizumab for use as add-on therapy in adults and children over six years of age with inadequately controlled severe persistent allergic IgE-mediated asthma who require continuous or frequent treatment with oral corticosteroids.
Objectives: To assess the effects of omalizumab versus placebo or conventional therapy for asthma in adults and children.
Search methods: We searched the Cochrane Airways Group Specialised Register of trials for potentially relevant studies. The most recent search was performed in June 2013. We also checked the reference lists of included trials and searched online trial registries and drug company websites.
Selection criteria: Randomised controlled trials examining anti-IgE administered in any manner for any duration. Trials with co-interventions were included, as long as they were the same in each arm.
Data collection and analysis: Two review authors independently assessed study quality and extracted and entered data. Three modes of administration were identified from the published literature: inhaled, intravenous and subcutaneous injection. The main focus of the updated review is subcutaneous administration, as this route is currently used in clinical practice. Subgroup analysis was performed by asthma severity. Data were extracted from published and unpublished sources.
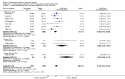
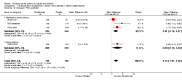
Main results: In all, 25 trials were included in the review, including 11 new studies since the last update, for a total of 19 that considered the efficacy of subcutaneous anti-IgE treatment as an adjunct to treatment with corticosteroids.For participants with moderate or severe asthma who were receiving background inhaled corticosteroid steroid (ICS) therapy, a significant advantage favoured subcutaneous omalizumab with regard to experiencing an asthma exacerbation (odds ratio (OR) 0.55, 95% confidence interval (CI) 0.42 to 0.60; ten studies, 3261 participants). This represents an absolute reduction from 26% for participants suffering an exacerbation on placebo to 16% on omalizumab, over 16 to 60 weeks. A significant benefit was noted for subcutaneous omalizumab versus placebo with regard to reducing hospitalisations (OR 0.16, 95% CI 0.06 to 0.42; four studies, 1824 participants), representing an absolute reduction in risk from 3% with placebo to 0.5% with omalizumab over 28 to 60 weeks. No separate data on hospitalisations were available for the severe asthma subgroup, and all of these data were reported for participants with the diagnosis of moderate to severe asthma. Participants treated with subcutaneous omalizumab were also significantly more likely to be able to withdraw their ICS completely than those treated with placebo (OR 2.50, 95% CI 2.00 to 3.13), and a small but statistically significant reduction in daily inhaled steroid dose was reported for omalizumab-treated participants compared with those given placebo (weighted mean difference (WMD) -118 mcg beclomethasone dipropionate (BDP) equivalent per day, 95% CI -154 to -84). However, no significant difference between omalizumab and placebo treatment groups was seen in the number of participants who were able to withdraw from oral corticosteroid (OCS) therapy (OR 1.18, 95% CI 0.53 to 2.63).Participants treated with subcutaneous omalizumab as an adjunct to treatment with corticosteroids required a small but significant reduction in rescue beta2-agonist medication compared with placebo (mean difference (MD) -0.39 puffs per day, 95% CI -0.55 to -0.24; nine studies, 3524 participants). This benefit was observed in both the moderate to severe (MD -0.58, 95% CI -0.84 to -0.31) and severe (MD -0.30, 95% CI -0.49 to -0.10) asthma subgroups on a background therapy of inhaled corticosteroids; however, no significant difference between subcutaneous omalizumab and placebo was noted for this outcome in participants with severe asthma who were receiving a background therapy of inhaled plus oral corticosteroids. Significantly fewer serious adverse events were reported in participants assigned to subcutaneous omalizumab than in those receiving placebo (OR 0.72, 95% CI 0.57 to 0.91; 15 studies, 5713 participants), but more injection site reactions were observed (from 5.6% with placebo to 9.1% with omalizumab).To reflect current clinical practice, discussion of the results is limited to subcutaneous use, and trials involving intravenous and inhaled routes have been archived.
Authors' conclusions: Omalizumab was effective in reducing asthma exacerbations and hospitalisations as an adjunctive therapy to inhaled steroids and during steroid tapering phases of clinical trials. Omalizumab was significantly more effective than placebo in increasing the numbers of participants who were able to reduce or withdraw their inhaled steroids. Omalizumab was generally well tolerated, although more injection site reactions were seen with omalizumab. Further assessment in paediatric populations is necessary, as is direct double-dummy comparison with ICS. Although subgroup analyses suggest that participants receiving prednisolone had better asthma control when they received omalizumab, it remains to be tested prospectively whether the addition of omalizumab has a prednisolone-sparing effect. It is also not clear whether there is a threshold level of baseline serum IgE for optimum efficacy of omalizumab. Given the high cost of the drug, identification of biomarkers predictive of response is of major importance for future research.
Conflict of interest statement
2013 update:
SW has received travel grants from AstraZeneca, GlaxoSmithKline, Schering‐Plough, Aventis Pharma and 3M.
SM has received support with travel costs and conference attendance from Novartis.
EHW has received research support from GlaxoSmithKline, AstraZeneca, Novartis, Boehringer and Schering‐Plough in the past but none in recent years.
PN has received research grants from GSK, AZ and Schering, and honoraria or travel grants from GSK, AZ, Merck, Novartis, Teva, BI and Cipla.He is listed on a patent for a sputum filtration device and has provided scientific advice for a university spin‐off company, Cellometrics Inc.
RN: none known.
Original version of the review: Michele Monteil has received travel grants from GlaxoSmithKline and Merck Sharpe & Dohme.
Figures





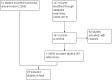






























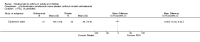




























Update of
-
Anti-IgE for chronic asthma in adults and children.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003559. doi: 10.1002/14651858.CD003559.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Jan 13;(1):CD003559. doi: 10.1002/14651858.CD003559.pub4. PMID: 16625585 Updated.
Comment in
-
Omalizumab decreases exacerbations and allows a step down in daily inhaled corticosteroid dose in adults and children with moderate-to-severe asthma.Evid Based Med. 2014 Aug;19(4):135. doi: 10.1136/eb-2014-101759. Epub 2014 Mar 13. Evid Based Med. 2014. PMID: 24627175 No abstract available.
References
References to studies included in this review
Bardelas 2012 {published data only}
-
- Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26‐week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. Journal of Asthma 2012;49(2):144‐52. - PubMed
-
- Figliomeni M, Kianifard F, Meng X. A 26‐week, randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the effect of omalizumab on markers of asthma impairment in patients with persistent allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB84. - PubMed
-
- NCT00870584. A 26‐Week Randomized, Double‐blind, Placebo‐Controlled, Multi‐Center Study to Evaluate the Effect of Omalizumab on Markers of Asthma Impairment in Patients With Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00870584 (accessed 7 January 2013). []
Boulet 1997 {published data only}
-
- Boulet L‐P, Chapman KR, Côté J, Kalra S, Bhagat R, Swystun VA, et al. Inhibitory effects of an anti‐IgE antibody E25 on allergen‐induced early asthmatic response. American Journal of Respiratory and Critical Care Medicine 1997;155:1835‐40. - PubMed
Busse 2001 {published and unpublished data}
-
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. - PubMed
-
- Busse W, Corren J, Lanier BQ, Mcalary M, Fowler‐Taylor A, Della Cioppa G, et al. Anti‐IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. Journal of Allergy and Clinical Immunology 2001;108(2):184‐90. - PubMed
-
- Finn A, Gross G, Bavel J, Lee T, Windom H, Everhard F, et al. Omalizumab improves asthma‐related quality of life in patients with severe allergic asthma. Journal of Allergy and Clinical Immunology 2003;111(2):278‐84. - PubMed
-
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsar... (accessed 7 January 2013):26‐78.
-
- Lanier RQ, Busse W, Corren J, Chervinsky P, Bernstein J, McAlary M, et al. Long‐term improvement in asthma control and exacerbation frequency is achieved with omalizumab (Xolair) in patients with moderate‐severe asthma. ATS International Conference; May 18‐23; San Francisco. 2001:185.
Busse 2011 {published data only}
-
- NCT00377572. Inner‐City Anti‐IgE Therapy for Asthma. www.clinicaltrials.gov/show/NCT00377572 (accessed 7 January 2013). []
Chanez 2010 {published data only}
-
- CIGE025AFR02. Double blind placebo controlled study to assess the expression of Fcϵ1 on blood basophils and dendritic cells in patients with uncontrolled severe persistent allergic asthma after a 16‐week omalizumab treatment. www.novctrd.com (accessed 2 October 2010).
-
- Chanez P, Contin‐Bordes C, Garcia G, Verkindre C, Didier A, Blay F, et al. Omalizumab‐induced decrease of FcRI expression in patients with severe allergic asthma. Respiratory Medicine 2010; Vol. 104, issue 11:1608‐17. - PubMed
-
- NCT00454051. Double Blind Placebo Controlled Study to Assess the Expression of IgE on Basophils and Dendritic Cells During Omalizumab Treatment. www.clinicaltrials.gov/show/NCT00454051 (accessed 7 January 2013). []
Djukanovic 2004 {unpublished data only}
-
- Djukanovic R, Wilson SJ, Kraft M, Jarjour N, Steel M, Chung KF, et al. Effect of treatment with anti‐IgE antibody (omalizumab) on airway inflammation in mild atopic asthma [abstract]. American Thoracic Society 99th International Conference; 2003 May 16‐21; Seattle C082.
-
- Djukanovic R, Wilson SJ, Kraft M, Jarjour N, Steel M, Chung KF, et al. Omalizumab, an anti‐IgE antibody, suppresses airway inflammation in mild allergic asthma via a reduction in mast cell surface‐associated interlukin‐4 [Abstract]. Allergy & Clinical Immunology International 2003, issue Suppl 1:Abstract No: 0‐17‐3.
-
- Djukanovic R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti‐immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. American Journal of Respiratory & Critical Care Medicine 2004;170(6):583‐93. - PubMed
Fahy 1997 {published data only}
-
- Fahy JV, Flemming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti‐IgE monoclonal antibody on the early‐ and late‐phase responses to allergen inhalation in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1828‐34. - PubMed
Fahy 1999 {published data only}
-
- Fahy JV, Cochroft DW, Boulet LP, Wong HH, Deschesnes F, Davis EE, et al. Effect of aerosolized anti‐IgE (E25) on airways responses to inhaled allergen in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine 1999;160(3):1023‐7. - PubMed
Garcia 2012 {published data only}
-
- Garcia G, Magnan A, Chiron R, Cecile C‐B, Berger P, Taille C. A randomized‐controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A6764. []
-
- Garcia G, Magnan A, Chiron R, Girodet P‐O, Gros VMH. A proof‐of‐concept randomized‐controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma [Abstract]. European Respiratory Journal 2012;40(Suppl 56):856s [4692]. [] - PubMed
Gevaert 2012 {published data only}
-
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and non‐allergic patients with nasal polyps and asthma [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl):AB69 [258]. - PubMed
-
- Gevaert P, Calus L, Zele T, Blomme K, Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients. Journal of Allergy and Clinical Immunology 2013;131(1):110‐116e1. [] - PubMed
Hanania 2011 {published data only}
-
- Condemi JJ, Hamilos DL, Hanania NA, Reyes‐Rivera I, Rosen KE, Wong D, et al. Efficacy and safety of omalizumab in patients with moderate‐to‐severe persistent asthma poorly controlled on high‐dose inhaled corticosteroids and long‐acting beta‐agonists: results of a phase III randomized controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6840.
-
- Dorenbaum A, Trzaskoma B, Haselkorn T, Mink D, Chen H, Solari P. Patient characteristics predictive of omalizumab response in EXTRA. Annals of Allergy, Asthma & Immunology 2012;109(Suppl):A54. []
-
- Hanania N, Condemi J, Hamilos D, Reyes‐Rivera I, Rosen KE, Wong D, et al. Omalizumab in patients with moderate‐to‐severe persistent asthma poorly controlled on high‐dose inhaled corticosteroids and long‐acting beta‐agonists: results of a phase IIIb randomized controlled trial [Abstract]. European Respiratory Society Annual Congress; September 18‐22; Barcelona. 2010:[E5487].
-
- Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes‐Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Annals of Internal Medicine 2011;154(4):573‐82. - PubMed
-
- Hanania NA, Wenzel S, Rosen K, Hsieh H‐J, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma. American Journal of Respiratory and Critical Care Medicine 2013;187(8):804‐11. [] - PubMed
Holgate 2004a {published and unpublished data}
-
- CIGE0250011E1. An open‐label extension to assess long‐term safety and tolerability of omalizumab treatment in adolescents and adults with severe allergic asthma who participated in the 32‐week core study. www.novctrd.com (accessed 10 February 2010).
-
- CIGE0250011E3. An open‐label extension study to assess long term safety and tolerability of omalizumab treat‐ment in adults and adolescents with severe allergic asthma who participated in the 52 week CIGE025 0011E2 study. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
-
- Chuchalin AG, Herbert J, Rolli M, Gao J, Resiner C. Long‐term safety and tolerability of omalizumab an anti‐IgE monoclonal antibody in patients with severe allergic asthma. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 421.
-
- Chung F, Holgate S, O'Brien J, Fox H, Thirlwell J. Inhaled corticosteroid dose reducing effect of omalizumab in patients with controlled severe asthma, according to usage of inhaled long acting beta‐agonist. American Academy of Allergy Asthma and Immunology 58th annual meeting. New York, New York, USA. March 1‐6. 2002:[726].
-
- Hebert J, Chuchalin A, Rolli M, Fox H. Long‐term safety and tolerability of omalizumab in adults with severe allergic asthma. American Thoracic Society Meeting 100th International Conference; 2004 May 21‐26; Orlando. 2004.
Holgate 2004b {published data only}
-
- CIGE0250011E1. An open‐label extension to assess long‐term safety and tolerability of omalizumab treatment in adolescents and adults with severe allergic asthma who participated in the 32‐week core study. www.novctrd.com (accessed 10 February 2010).
-
- CIGE0250011E3. An open‐label extension study to assess long term safety and tolerability of omalizumab treatment in adults and adolescents with severe allergic asthma who participated in the 52 week CIGE025 0011E2 study. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
-
- Chuchalin AG, Herbert J, Rolli M, Gao J, Resiner C. Long‐term safety and tolerability of omalizumab an anti‐IgE monoclonal antibody in patients with severe allergic asthma. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 421.
-
- Chung F, Holgate S, O'Brien J, Fox H, Thirlwell J. Inhaled corticosteroid dose reducing effect of omalizumab in patients with controlled severe asthma, according to usage of inhaled long acting beta‐agonist. American Academy of Allergy Asthma and Immunology 58th Annual Meeting. New York, New York, USA. March 1‐6, 2002 2002:[726].
-
- Hebert J, Chuchalin A, Rolli M, Fox H. Long‐term safety and tolerability of omalizumab in adults with severe allergic asthma. American Thoracic Society Meeting 100th International Conference; 2004 May 21 ‐ 26; Orlando. 2004.
INNOVATE {unpublished data only}
-
- Berger W, Humbert M, Leighton T, Turk F, Hedgecock S, Ayre G, et al. Asthma patients judged by the physician to have responded to add‐on omalizumab therapy have a greater percentage of symptom‐free days. Proceedings of the American Thoracic Society; 2006 May 19‐24; San Diego. 2006:A591.
-
- Bleecker E, Rubinfield A, Hedgecock S, Fox H, Surrey K, Reisner C. Add‐on omalizumab therapy significantly improves symptom control and reduces exacerbations in patients with inadequately controlled severe persistent asthma despite GINA 2002 Step 4 therapy irrespective of maintenance oral corticosteroid (OCS) use: INNOVATE. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:B36; Poster: G51.
-
- Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respiratory Medicine 2007;101(7):1483‐92. - PubMed
-
- Brown R, Turk F, Groot M, Dale P. Cost effectiveness of omalizumab in patients with severe persistent allergic (IgE‐mediated) asthma adaptation of INNOVATE and ETOPA data to the Netherlands. European Respiratory Journal 2007;30(Suppl 51):194s.
-
- Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005;60(3):309‐16. - PubMed
Lanier 2009 {published data only}
-
- Bousquet J, Kulus M, Fox H, Blogg M, Fowler‐Taylor A, Fernandez‐Vidaurre C. Omalizumab therapy reduces asthma exacerbations in children with severe allergic (ige‐mediated) asthma irrespective of lung function at baseline [Abstract].. European Respiratory Society Annual Congress; September 12‐16; Vienna. 2009:3281.
-
- Bridges T, Hebert J, Fowler‐Taylor A, Fernandez‐Vidaurre C, Berhane I. Omalizumab reduces asthma exacerbations in children (6‐<12 years) with moderate‐to‐severe allergic (IgE‐mediated) asthma irrespective of baseline LABA use [Abstract]. European Respiratory Society Annual Congress; September 12‐16; Vienna, Austria. 2009:P1219.
-
- CIGE025AIA05. A 1 year, randomized, double blind, parallel‐group, placebo‐controlled, multicenter evaluation of efficacy, safety, pharmacokinetics and pharmacodynamics of omalizumab in children (6 ‐ <12 years) with moderate‐severe, persistent, inadequately controlled allergic asthma. www.novctrd.com (accessed 10 February 2010).
-
- Kulus M, Bridges T, Fowler‐Taylor A, Blogg M, Jimenez P. A randomized controlled study of omalizumab in children with moderate to severe persistent allergic asthma [Abstract]. European Respiratory Society Annual Congress; 2008 October 4‐8; Berlin.
-
- Kulus M, Hébert J, Garcia E, Fowler Taylor A, Fernandez Vidaurre C, Blogg M. Omalizumab in children with inadequately controlled severe allergic (IgE‐mediated) asthma. Current Medical Research and Opinion 2010;26(6):1285‐93. - PubMed
Massanari 2010 {published data only}
-
- CIGE025A US23. A 26‐week, randomized, double‐blind, parallel‐group, placebo‐controlled, multi‐center study to evaluate the effect of omalizumab on improving the tolerability of specific immunotherapy in patients with at least moderate persistent allergic asthma inadequately controlled with inhaled corticosteroids. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
-
- Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. Journal of Allergy and Clinical Immunology 2010;125(2):383‐9. - PubMed
-
- NCT00267202. A 26‐Week, Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled, Multi‐Center Study to Evaluate the Effect of Omalizumab on Improving the Tolerability of Specific Immunotherapy in Patients With at Least Moderate Persistent Allergic Asthma Inadequately Controlled With Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00267202 (accessed 7 January 2013). []
Milgrom 1999 {published data only}
-
- Metzger WJ, Fick RB, Bush RK, Busse W, Casale T, Chodosh S, et al. Corticosteroid (CS) withdrawal in a study of recombinant humanized monoclonal antibody to IgE (rhu MAbE25). Journal of Allergy and Clinical Immunology 1998;101(1):231.
-
- Milgrom H, Fick RB, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti‐IgE antibody. New England Journal of Medicine 1999;341(26):1966‐73. - PubMed
Milgrom 2001 {published data only}
-
- Berger W, Gupta N, McAlary M, Fowler‐Taylor A. Evaluation of long‐term safety of the anti‐IgE antibody, omalizumab, in children with allergic asthma. Annals of Allergy Asthma & Immunology 2003;91(2):182‐8. - PubMed
-
- Buhl R, Soler M, Fox H, Ashby M, McAlary M, Cooper J, et al. Omalizumab (Xolair, rhumab‐e25) decreases hospitalisations due to serious asthma exacerbations. Proceedings of the ATS 97th International conference; 2001 May 18‐23; San Francisco. 2001:186.
-
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 January 2013):79‐98.
-
- Lemanske RF, Nayak A, McAlary M, Everhard F, Fowler‐Taylor A, Gupta N. Omalizumab improves asthma‐related quality of life in children with allergic asthma. Pediatrics 2002;110(5):e55. - PubMed
-
- Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti‐immunoglobulin E antibody (omalizumab). Pediatrics 2001;108:36. - PubMed
NCT00096954 {published data only}
-
- NCT00096954. A Prospective, Randomized, Double‐Blind Study of the Efficacy of Omalizumab (Xolair) in Atopic Asthmatics With Good Lung Capacity Who Remain Difficult to Treat (EXACT). Http://clinicaltrials.gov/show/NCT00096954 (accessed 7 January 2013). []
NCT01007149 {published data only}
-
- CIGE025AFR05 A 16‐Week Treatment, Multicenter, Randomized, Double Blind, Placebo‐Controlled, Parallel‐Group Study to Assess the Effect of Omalizumab on the Expression of FcεRI Receptors of Blood Basophils and Dendritic Cells in Patients With Severe Persistent Non‐Atopic Asthma, Uncontrolled Despite Optimal Therapy. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
-
- NCT01007149. A 16‐Week Treatment, Multicenter, Randomized, Double Blind, Placebo‐Controlled, Parallel‐Group Study to Assess the Effect of Omalizumab on the Expression of FcεRI Receptors of Blood Basophils and Dendritic Cells in Patients With Severe Persistent Non‐Atopic Asthma, Uncontrolled Despite Optimal Therapy. www.clinicaltrials.gov/show/NCT01007149 (accessed 7 January 2013). []
Ohta 2009 {published data only}
-
- CIGE025A1304. Multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study with a 16‐weektreatment phase to determine the efficacy, safety and tolerability of subcutaneous omalizumab at a dose of at least 0.016mg/kg/IgE[IU/ml] every 2 or 4 weeks for the treatment of patients with moderate to severe bronchial asthma. The trial consisted of 3 periods: a 2‐week screening period, a16‐week treatment period and a 24‐week post‐treatment follow‐up period. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
-
- NCT00232050. Study of Omalizumab in Moderate to Severe Bronchial Asthma. www.clinicaltrials.gov/show/NCT00232050 (accessed 7 January 2013). []
-
- Ohta K, Miyamoto T, Amagasaki T, Yamamoto M. Efficacy and safety of omalizumab in an Asian population with moderate‐to‐severe persistent asthma. Respirology 2009;14(8):1156‐65. - PubMed
-
- Ohta K, Miyamoto T, Yamamoto M, Fox H, Blogg M. Omalizumab improves lung function in asthmatic smokers with severe persistent allergic asthma. American Thoracic Society International Conference; 18‐23 May; San Francisco. 2007:Poster F76.
Prieto 2006 {published data only}
-
- Bruno L, Prieto L, Gutierrez V, Colas C, Tabar AI, Perez‐Frances, et al. Effect of omalizumab on adenosine 5'‐monophosphate responsiveness in allergic asthma [Abstract]. XIX World Allergy Organization Congress; Munich. 2005:Abstract 306.
-
- CIGE025AES01. Effect of omalizumab on bronchial responsiveness to adenosine 5'‐monophosphate (AMP) in patients with asthma. www.novctrd.com (accessed 2 October 2010).
-
- Prieto L, Gutierrez V, Colas C, Tabar A, Perez‐Frances C, Bruno L, et al. Effect of omalizumab on adenosine 5'‐monophosphate responsiveness in subjects with allergic asthma. International Archives of Allergy and Immunology 2006;139(2):122‐31. - PubMed
SOLAR {published data only}
-
- Boulet LP, Canonica GW, Dahl R, Hedgecock S, Blogg M, Surrey K, et al. Omalizumab, an Anti‐IgE antibody, provides parallel improvements in symptoms of allergic asthma and perennial allergic rhinitis in patients with both diseases: the SOLAR study. Chest 2003;124(4):105s.
-
- CIGE025A2304. A phase IIIb, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study with a 28‐week treatment phase to determine the efficacy, safety and tolerability of subcutaneous omalizumab for the treatment of 12‐75 year‐old patients with comorbid moderate‐to‐severe allergic asthma and perennial allergic rhinitis. www.novctrd.com (accessed 10 February 2010).
-
- Dahl R, Ayres J, Hedgecock S, Blogg M, Surrey K, Fox H. Efficacy of omalizumab, an anti‐IgE antibody, in patients with concomitant moderate‐severe allergic asthma and persistent allergic rhinitis [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S37.
-
- Hanf G, Noga O, Brachmann I, Kleine‐Tebbe J, Rosseau S, Suttorp N, et al. Omalizumab (rhuMAb‐E25) inhibits the IgE in vitro release of stimulated PBMC of allergic asthmatics. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:B36; Poster: G58.
-
- Harnest U, Boulet L, Hedgecock S, Blogg M, Surrey K, Fox H. Omalizumab, an anti‐IgE antibody, improves both asthma and rhinitis‐related quality of life in patients with concomitant moderate‐severe disease [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S175.
Solèr 2001 {published data only}
-
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. - PubMed
-
- Buhl R, Hanf G, Soler M, Bensch G, Wolfe J, Everhard F, et al. The anti‐IgE antibody omalizumab improves asthma‐related quality of life in patients with allergic asthma. European Respiratory Journal 2002;20(5):1088‐94. - PubMed
-
- Buhl R, Solèr M, Matz J, Townley R, O'Brien J, Noga O, et al. Omaliziumab provides long‐term control in patients with moderate‐to‐severe allergic asthma. European Respiratory Journal 2002;20:73‐8. - PubMed
-
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 January 2013):26‐78.
-
- Luskin AT, Kosinski M, Bresnahan BW, Ashby M, Wong DA. Symptom control and improved functioning: the effect of omalizumab on Asthma‐Related Quality of Life (ARQL). Journal of Asthma 2005;42(10):823‐7. - PubMed
van Rensen 2009 {published data only}
-
- Rabe KF, Rensen ELJ, Evertse CE, Schadewijk WAA, Wijngaarden S, Ayre G, et al. Anti‐IgE induced changes in bronchial high affinity IgE receptor expressing cells and eosinophils in biopsies in patients with asthma role of DC‐SIGN [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A569.
-
- Rensen EL, Evertse CE, Gauw SA, Ayre G, Sterk PJ, Rabe KF. Anti‐IgE treatment improves lung function in patients with mild persistent allergic asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S9.
-
- Rensen EL, Evertse CE, Schadewijk WA, Wijngaarden S, Ayre G, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti‐IgE treatment. Allergy 2009;64(1):72‐80. - PubMed
-
- Rensen ELJ, Evertse CE, Schadewijk WAA, Veen H, Timmers MC. Anti‐IgE omalizumab treatment reduces allergen‐induced eosinophilia in biopsies and sputum in patients with asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:C12.
References to studies excluded from this review
Anonymous 2000 {published data only}
-
- Anonymous. Anti‐IgE for allergic asthma. Hospital Practice 2000;24(2):27‐8.
Anonymous 2000b {published data only}
-
- Anonymous. Anti‐IgE antibody reduces need for glucocorticosteroids [Anti‐IgE Antikörper: Bedarf an Glucocorticoiden sinkt]. Deutsche Apotheker Zeitung 2000;140(12):1289‐92.
Anonymous 2003 {published data only}
-
- Anonymous. Omalizumab appears effective in patients with poorly controlled allergic asthma. Formulary 2003;38(4):197, 203.
Ayars 2011 {published data only}
-
- Ayars AG, Altman LC, Potter‐Perigo S, Wight TN, Nair P. Sputum hyaluronan as a biomarker of airway remodeling in severe asthma [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB8.
Ayars 2013 {published data only}
Babu 2001 {published data only}
-
- Babu KS, Arshad SH, Holgate ST. Anti‐IgE treatment: an update. Allergy 2001;56:1121‐8. - PubMed
Beeh 2006 {published data only}
-
- Beeh K‐M, Pereno R, Chen H, Jimenez P. Adding omalizumab to high dose ICS and LABA significantly improves quality of life in patients with severe persistent allergic asthma [Abstract]. European Respiratory Journal 2006;28(Suppl 50):440s.
Berger 2002 {published data only}
-
- Berger WE. Monoclonal anti‐IgE antibody: a novel therapy for allergic airways disease. Annals of Allergy and Immunology 2002;88:152‐60. - PubMed
Bisberg 1996 {unpublished data only}
-
- Bisberg D, Froehlich J, Schoenhoff M, Mendelson J. Multiple administrations of the Anti‐IgE recombinant humanized monoclonal antibody E25 (rhuMAb‐E25) reduces free IgE levels in a dose dependent manner in adolescents and children with moderate to severe allergic asthma. Journal of Clinical Pharmacology 1996;36:859.
Blanken 2013 {published data only}
-
- Blanken MO, Rovers MM, Molenaar JM, Winkler‐Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy. New England Journal of Medicine 2013;368(19):1791‐9. [] - PubMed
Bousquet 2010 {published data only}
-
- Bousquet J, Smith N, Peckitt C, Maykut R, Peachey G. The effectiveness of different clinical measures in evaluating response to omalizumab. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:E3764.
Bousquet 2011 {published data only}
-
- Bousquet J, Siergiejko Z, Swiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE‐mediated) asthma. Allergy 2011;66(5):671‐8. - PubMed
-
- Magyar P, Peckitt C, Maykut R, Peachey G. Persistency of treatment response to omalizumab in patients with severe allergic (ige‐mediated) asthma [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna, Austria. 2009:[E1870].
-
- NCT00264849. A Randomized, Open Label, Parallel‐group, International, Multicenter Study Evaluating Persistency of Response to Omalizumab During 32 Weeks Treatment Given as Add on to Optimized Asthma Therapy in Adult and Adolescent Patients With Severe Persistent Allergic Asthma, Who Remain Inadequately Controlled Despite GINA (2004) Step 4 Therapy. www.clinicaltrials.gov/show/NCT00264849 (accessed 7 January 2013). []
-
- Siergiejko Z, Swiebocka E, Peckitt C, Maykut R, Peachey G. Omalizumab improves quality of life in adults and adolescents (≥12 years) with uncontrolled severe allergic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6651.
-
- Siergiejko Z, Swiebocka E, Peckitt C, Maykut R, Peachey G. Omalizumab reduces healthcare resource utilization in adults and adolescents (≥12 years) with uncontrolled severe allergic asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A5404.
Bruselle 2009 {published data only}
-
- Brusselle G, Michils A, Louis R, Dupont L, Maele B, Delobbe A, et al. "Real‐life" effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respiratory Medicine 2009;103(11):1633‐42. - PubMed
Buhl 2001 {unpublished data only}
-
- Buhl R, Soler M, Fox H, Ashby M, McAlary M, Cooper J, et al. Omalizumab (XOLAIR, rhu‐MAb‐E25), decreases hospitalisations die to serious asthma exacerbations. Proceedings of the Annual Thoracic Society 97th International Conference, May 18‐23; San Francisco. 2001:186.
Busse 2013 {published data only}
-
- Busse WW, Holgate ST, Kerwin EM, Chon Y, Feng JY, Lin JH, et al. A randomized, double‐blind, placebo‐controlled, multiple‐dose study to evaluate the safety, tolerability, and efficacy of brodalumab (AMG 827) in subjects with moderate to severe asthma. Journal of Allergy and Clinical Immunology 2013;131(2):AB230 [817]. []
Castro 2011 {published data only}
-
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Annals of Allergy Asthma and Immunology 2011;107(1):65‐70. - PubMed
Chipps 2009 {published data only}
-
- Chipps B, Kianifard F, Fernandez VC, Massanari M. Effect of omalizumab on peripheral blood eosinophils in children with moderate‐severe persistent allergic asthma [Abstract]. Chest 2009;136(4):35S.
CIGE025A1305 {unpublished data only}
-
- CIGE025A1305. Comparative study of IGE025 with suplatast tosilate (IPD) in patient with Japanese cedar pollen‐induced seasonal allergic rhinitis (SAR). www.novctrd.com (accessed 10 February 2010).
CIGE025A1306 {published data only}
-
- CIGE025A1306. Open‐label study of IGE025 in patients with Japanese cedar pollen‐induced seasonal allergic rhinitis (SAR) for second season administration. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
CIGE025A1307 {unpublished data only}
-
- CIGE025A1307. Long‐term study of IGE025 in moderate to severe bronchial asthma. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
CIGE025A2208 {published data only}
-
- CIGE025A2208. Multi‐center, open‐label, multiple dose study in mild to moderate asthmatics (with IgE/body weight combinations above that in the SmPC dosing table) to determine safety, tolerability, pharmacokinetics, and pharmacodynamics of omalizumab. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 January 2013).
CIGE025A2303 {unpublished data only}
-
- CIGE025A2303. A 52 week treatment, multicenter, randomized, double‐blind, parallel‐group, placebo controlled study to investigate the effect of omalizumab (rhuMAb‐E25) on intestinal geohelminth reinfection in adolescent/ adult patients with allergic asthma and/or perennial allergic rhinitis previously treated with an anti intestinal geohelminth treatment regimen. www.novctrd.com (accessed 10 February 2010).
CIGE025AUS23 {unpublished data only}
-
- CIGE025AUS23. A 26‐week, randomized, double‐blind, parallel‐group, placebo‐controlled, multi‐center study to evaluate the effect of omalizumab on improving the tolerability of specific immunotherapy in patients with at least moderate persistent allergic asthma inadequately controlled with inhaled corticosteroids. www.novartisclinicaltrials.com (accessed 12 March 2009).
Corren 2010 {published data only}
-
- Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL‐4Ralpha antagonist, in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(8):788‐96. - PubMed
Corren 2011 {published data only}
-
- Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. New England Journal of Medicine 2011;365(12):1088‐98. - PubMed
Corren 2011a {published data only}
-
- Corren J, Wood R, Patel D, Zhu J, Yegin A, Dhillon G, et al. Omalizumab efficacy following cat allergen exposure: Rresults from a randomized, double‐blind, placebo‐controlled, parallel‐group study in patients with moderate asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[E3962].
-
- Corren J, Wood RA, Patel D, Zhu J, Fish J E. A randomized, double‐blind, placebo‐controlled, parallel‐group study of the efficacy of omalizumab in prevention of bronchoconstriction following environmental aeroallergen exposure [Abstract]. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB72.
-
- Corren J, Wood RA, Patel D, Zhu J, Yegin A, Dhillon G, et al. Effects of omalizumab on changes in pulmonary function induced by controlled cat room challenge. Journal of Allergy and Clinical Immunology 2011;127(2):398‐405. - PubMed
-
- NCT00495612. A Phase IV, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study of the Efficacy of Omalizumab in Preventing Bronchoconstriction Following Environmental Cat Dander Exposure in Patients With Cat Dander‐Induced Asthma. www.clinicaltrials.gov/show/NCT00495612 (accessed 7 January 2013). []
Demoly 1997 {published data only}
-
- Demoly P, Bousquet J. Rhu‐MAb‐E25 reduces but does not abrogate the early and late phase reaction following allergen bronchial challenge. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1825‐7. - PubMed
Eckman 2010 {published data only}
Emmrich 2001 {published data only}
-
- Emmrich P, Kruse K, Reinhardt D. Anti‐IgE antibodies in the treatment of acute asthma [Asthmatherapie mit Anti‐IgE Antikörpern]. Monatsschrift für Kinderheilkunde 2001;149(5):506.
ETOPA {published and unpublished data}
-
- Ayres G, Anthonissen C, Martin C, Turk F, Thomas K. Assessment of a responder identification treatment algorithm for omalizumab is a naturalistic setting. European Respiratory Journal 2007;30(Suppl 51):623s.
-
- Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti‐immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate‐to‐severe) allergic asthma. Allergy 2004;59(7):701‐8. - PubMed
-
- Ayres JG, Niven R, Ayre G, Blogg M, Fox H. Omalizumab reduces the rate asthma deterioration related incidents in patients with poorly controlled allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S202.
-
- Bousquet J, Ayres G, Blogg M. Omalizumab added to best standard care reduces exacerbations in patients with severe persistent asthma according to GINA 2002 classification [Abstract]. European Respiratory Journal 2004;24(Suppl 48):220s.
-
- Bousquet J, Niven R, Ayre G, Fox H, Bogg M. Efficacy of omalizumab in patients with moderate to severe allergic asthma that is poorly controlled on GINA (1998) treatment step 3 or 4 [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: 1389.
Fernandez 2005 {published data only}
-
- Fernandez C, Busse W, Resiner C, Gupta N. Clinical data do not suggest a casual relationship between omalizumab therapy and cancer. American Thoracic Society 2005 International Conference; May 20‐25; San Diego. 2005:[B36; Poster: G50].
Frew 1998 {published data only}
Gauvreau 2012 {published data only}
-
- Gauvreau G, Boulet L‐P, Cockcroft D, Davis B, Fitzgerald M, Leigh R, et al. Effects of anti‐M1 prime monoclonal antibody, MEMP1972A following allergen challenge in patients with mild asthma [Abstract]. European Respiratory Journal 2012;40(Suppl 56):547s [3087]. []
Gauvreau 2012a {published data only}
-
- Gauvreau G, Boulet L‐P, Cockcroft DW, Davis B, Fitzgerald MJ, Leigh R. Effect of an anti‐M1 prime monoclonal antibody, MEMP1972A, in a phase II proof‐of‐activity allergen challenge study in patients with mild asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012; Vol. 185, issue Meeting Abstracts:A6793. []
Gober 2008 {published data only}
-
- Gober LM, Sterba PM, Eckman JA, Saini SS. Effect of anti‐IgE (omalizumab) in chronic idiopathic urticaria (CIU) patients [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S147.
Gossage 2010 {published data only}
-
- Gossage D, Geba G, Gillen A, Le C, Molfino N. A multiple ascending subcutaneous (SC) dose study of MEDI‐563, a humanized anti‐IL‐5R? monoclonal antibody, in adult asthmatics. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P1177].
Gossage 2012 {published data only}
-
- Gossage DL, Laviolette M, Gauvreau GM, Leigh R, Kolbeck R, Wu Y. Depletion of airway eosinophils by benralizumab an anti‐IL5 receptor alpha monoclonal antibody [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A3961. []
Hanania 2011a {published data only}
-
- Hanania NA, Lemanske RFJr, Korenblat PE, Arron JR, Harris JM, Su Z, et al. Efficacy of an anti‐IL13 monoclonal antibody, lebrikizumab, in adults with inadequately controlled asthma is enhanced in those with high periostin levels. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:608s [3426].
Hendeles 2007 {unpublished data only}
-
- Hendeles L, Khan Y, Massanari M, Spencer T, Shuster J, Chesrown S. The effect of omalizumab on airway responsiveness to adenosine in asthma patients with poor adherence to inhaled steroids. American Thoracic Society International Conference; May 18‐23; San Francisco. 2007:Poster #418.
-
- NCT00133042. The Effect of Omalizumab on Airway Responsiveness to Adenosine in Patients With Poorly Controlled Asthma. www.clinicaltrials.gov (accessed 10 February 2010).
Hodsman 2013 {published data only}
Holgate 2001 {published data only}
-
- Holgate S, Bousquet J, Wenzel S, Fox H, Liu J, Castellsague J. Efficacy of omalizumab, an anti‐immunoglobulin E antibody, in patients at high risk of serious asthma‐related morbidity and mortality. Current Medical Research and Opinion 2001;17(4):233‐40. - PubMed
Hoshino 2011 {published data only}
-
- Hoshino M. Effects of add‐on omalizumab therapy on airway wall thickening in severe persistent asthma. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam 2011;38(55):23s [P267].
-
- Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti‐immunoglobulin E antibody, on airway wall thickening in asthma.. Respiration 2012;83:520‐8. - PubMed
Hughes 2000 {published data only}
-
- Hughes TD. Anti‐IgE antibody may help treat some asthma patients. Journal of the American Medical Association 2000;284(22):2859‐60. - PubMed
Johansson 2009 {published data only}
-
- Johansson SG, Nopp A, Oman H, Ankerst J, Cardell LO, Gronneberg R, et al. The size of the disease relevant IgE antibody fraction in relation to 'total‐IgE' predicts the efficacy of anti‐IgE (Xolair) treatment. Allergy 2009;64(10):1472‐7. - PubMed
Kamin 2010 {published data only}
-
- Kamin W, Kopp M V, Erdnuess F, Schauer U, Zielen S, Wahn U. Safety of anti‐IgE treatment with omalizumab in children with seasonal allergic rhinitis undergoing specific immunotherapy simultaneously. Pediatric Allergy and Immunology 2010;21(1 Pt 2):e160‐5. - PubMed
Karpel 2010 {published data only}
-
- Karpel J, Massanari M, Geba GP, Kianifard F, Inhaber N, Zeldin RK. Effectiveness of omalizumab in reducing corticosteroid burden in patients with moderate to severe persistent allergic asthma. Annals of Allergy Asthma & Immunology 2010;105(6):465‐70. - PubMed
Kenyon 2011 {published data only}
-
- Kenyon NJ, Last MA, Bratt JM, Kwan VW, O'Roark E, Linderholm A. L‐arginine supplementation and metabolism in asthma. Pharmaceuticals 2011;4(1):187‐201.
Kopp 2009 {published data only}
-
- CIGE025ADE03. A randomized, 20 week, double‐blind, placebo‐controlled, parallel‐group, multiple‐dose, multicenter study to assess the efficacy and safety of omalizumab in combination with Depigoid, versus Depigoid only, in adult and adolescent patients with seasonal allergic asthma and comorbid seasonal allergic rhinoconjunctivitis—open‐label Depigoid monotherapy extension periods 2007and 2008. www.novctrd.com (accessed 10 February 2010).
-
- Kopp MV, Hamelmann E, Zielen S, Kamin W, Bergmann KC, Sieder C, et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co‐morbid seasonal allergic asthma. Clinical and Experimental Allergy 2009;39(2):271‐9. - PubMed
-
- NCT00396409. A Randomized, 20 Week, Double‐blind, Placebo‐controlled, Parallel‐group, Multiple‐dose, Multicenter Study to Assess the Efficacy and Safety of Omalizumab in Combination With Depigoid, Versus Depigoid Only, in Adult and Adolescent Patients With Seasonal Allergic Asthma and Comorbid Seasonal Allergic Rhinoconjunctivitis—Open‐label Depigoid Monotherapy Extension Periods 2007 and 2008. www.clinicaltrials.gov/show/NCT00396409 (accessed 7 January 2013). []
Lanier 2010 {published data only}
-
- Lanier B, Fowler Taylor A, Vidaurre CF, Blogg M. Number needed to treat (NNT) to prevent one exacerbation per year with omalizumab (OMA) In children with inadequately controlled allergic (IgE‐mediated) asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[P2640].
Leynadier 2004 {published data only}
-
- Leynadier F, Doudou O, Gaouar H, Gros V, Bourdeix I, Guyomarch‐Cocco L, et al. Effect of omalizumab in health care workers with occupational latex allergy. Journal of Allergy and Clinical Immunology 2004;113(2):360‐1. - PubMed
Lobo 2007 {published data only}
-
- Lobo ES, Revicki D, Grant W, Turk F, Massanari M. Assessment of the psychometric properties of the paediatric asthma quality of life questionnaire (PAQLQ) in moderate to severe pediatric asthma patients [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S151.
Massanari 2008 {published data only}
-
- Massanari M, Jimenez P, Kianifard F, Maykut R, Zeldin R. The omalizumab associated decrease in peripheral blood eosinophils in moderate severe IgE mediated asthma is sustained following inhaled steroid dose reduction [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A105.
Massanari 2009 {published data only}
-
- Massanari M, Kianifard F, Zeldin RK, Geba GP. Efficacy of omalizumab in cat‐allergic patients with moderate‐to‐severe persistent asthma. Allergy and Asthma Proceedings 2009;30(5):534‐9. - PubMed
-
- Massanari M, Sacco P, Kianifard F, Maykut R, Zeldin R. Addition of omalizumab improved functional health status in patients with impaired quality of life associated with moderate to severe persistent allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S154.
Maykut 2008 {published data only}
-
- Maykut R, Massanari M, Kianifard F, Zeldin R. Effect of omalizumab on asthma control and quality of life in patients with moderate severe persistent IgE‐mediated asthma and allergy to house dust mite [Abstract]. Journal of Allergy and Clinical Immunology 2008;21(2 Suppl 1):S157.
McClintock 2012 {published data only}
-
- McClintock D, Corren J, Hanania NA, Mosesova S, Lal P, Arron JR. Lebrikizumab, an anti‐IL‐13 monoclonal antibody, reduces severe asthma exacerbations over 32 weeks in adults with inadequately controlled asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A3959. []
Milgrom 2007 {published data only}
-
- Milgrom H, Massanari M, Maykut RJ, Kianifard F, Zeldin RK, Geba GP. Addition of omalizumab reduces school absenteeism in children with moderate to severe persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S150.
Milgrom 2009 {published data only}
-
- Milgrom H, Fink J, Fowler‐Taylor A, Fernandez Vidaurre C, Blogg M. Safety and tolerability of omalizumab in children with inadequately controlled moderate‐to‐severe allergic (IgE‐mediated) asthma [Abstract]. Thorax 2009;64(Suppl IV):A18 [S31].
Milgrom 2011 {published data only}
-
- Milgrom H, Fowler‐Taylor A, Vidaurre CF, Jayawardene S. Safety and tolerability of omalizumab in children with allergic (IgE‐mediated) asthma. Current Medical Research and Opinion 2011;27(1):163‐9. - PubMed
Molfino 2013 {published data only}
-
- Molfino NA, Bardin PG, Thompson PJ, Luckey A, Yarranton G. A randomized placebo‐controlled safety and pharmacodynamic study of KB002, a chimeric anti‐GM‐CSF monoclonal antibody, in patients with asthma. Journal of Allergy and Clinical Immunology 2013;131(2):AB229 [814]. []
Moulton 2000 {published data only}
-
- Moulton D. Anti‐IgE asthma treatment reduces corticosteroid use. Canadian Medical Association 2000;162(6):864.
NCT00109187 {published data only}
-
- NCT00109187. Open‐Label Extension Study II of Xolair (Omalizumab) in Moderate to Severe, Persistent Asthma Subjects Who Completed Study Q2143g (ALTO). www.clinicaltrials.gov/show/NCT00109187 (accessed 7 January 2013). []
NCT00109200 {published data only}
-
- NCT00109200. A Continued Access Protocol to Provide Xolair® (Omalizumab) to Subjects With Severe Allergic Asthma Who Have Received Xolair Treatment in a Previous Investigational Study. www.clinicaltrials.gov/show/NCT00109200 (accessed 7 January 2013). []
NCT00133042 {published data only}
-
- The Effect of Omalizumab on Airway Responsiveness to Adenosine in Patients With Poorly Controlled Asthma. http://ClinicalTrials.gov/show/NCT00133042 (accessed 7 January 2013).
NCT00180011 {published data only}
-
- NCT00180011. Efficacy of Omalizumab as Add on Therapy for Minority Patients With Moderate to Severe Asthma. www.clinicaltrials.gov/show/NCT00180011 (accessed 7 January 2013). []
NCT00189228 {published data only}
-
- NCT00189228. Not a Drug Trial. We Are Using Anti‐IgE to Examine the Role of Pulmonary Mast Cells in Asthma. www.clinicaltrials.gov/show/NCT00189228 (accessed 7 January 2013). []
NCT00201097 {published data only}
-
- NCT00201097. Immune Dysregulation in Allergic Asthma. www.clinicaltrials.gov/show/NCT00201097 (accessed 7 January 2013). []
NCT00219323 {published data only}
-
- NCT00219323. Long‐Term Study of IGE025 in Moderate to Severe Bronchial Asthma. www.clinicaltrials.gov/show/NCT00219323 (accessed 7 January 2013). []
NCT00242359 {unpublished data only}
-
- Bernstein JA. A Pilot Study Investigating the Effect of Omalizumab (Xolair) in Work‐Related Animal Induced Asthma. http://clinicaltrials.gov/show/NCT00242359 (accessed 7 January 2013).
NCT00283504 {published data only}
-
- NCT00283504. A Description of Inflammatory Cell Types in Moderate to Severe Pediatric Asthma: Eosinophilic and Non Eosinophilic Sputum Markers While on Anti‐IgE Therapy (Xolair). www.clinicaltrials.gov/show/NCT00283504 (accessed 7 January 2013). []
NCT00287378 {published data only}
-
- NCT00287378. Effect of Ozone on Airway Inflammation in Allergic Asthmatics Treated With Omalizumab. www.clinicaltrials.gov/show/NCT00287378 (accessed 7 January 2013). []
NCT00401596 {published data only}
-
- NCT00401596. A Multicenter, Randomized, Controlled, Open‐Label Study to Evaluate the Safety of Xolair in Moderate to Severe Persistent Asthma Subjects Already Treated With Other Therapies (ALTO). www.clinicaltrials.gov/show/NCT00401596 (accessed 7 January 2013). []
NCT00434434 {published data only}
-
- NCT00434434. A Phase II, Multicenter, Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled Study to Evaluate the Efficacy and Safety of Lyophilized and Aged Liquid Omalizumab in the Prevention of Allergen‐Induced Airway Obstruction in Adults With Mild Allergic Asthma. www.clinicaltrials.gov/show/NCT00434434 (accessed 7 January 2013). []
NCT00482248 {published data only}
-
- NCT00482248. An Open‐label Extension Study to Assess Long Term Safety and Tolerability of Omalizumab Treatment in Adults and Adolescents With Severe Allergic Asthma Who Participated in the 52 Week CIGE250011E2 Study. www.clinicaltrials.gov/show/NCT00482248 (accessed 7 January 2013). []
NCT00482508 {published data only}
-
- NCT00482508. A One Year Open‐label Extension Study to Assess Long Term Safety and Tolerability of Omalizumab Treatment in Poorly Controlled Moderate to Severe Allergic Asthma Patients Who Participated in the 52‐week CIGE24IA04E1 Study. www.clinicaltrials.gov/show/NCT00482508 (accessed 7 January 2013). []
NCT00500539 {published data only}
-
- NCT00500539. An Open Label, Single Arm Study to Assess the Safety and Immunogenicity of Omalizumab Liquid Administered Subcutaneously to Male and Female Adolescents and Adults With Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00500539 (accessed 7 January 2013). []
NCT00546143 {published data only}
-
- NCT00546143. Multi‐Center, Open‐Label, Multiple Dose Study in Mild to Moderate Asthmatics (With IgE/Body Weight Combinations Above That in the SmPC Dosing Table) to Determine Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Omalizumab. www.clinicaltrials.gov/show/NCT00546143 (accessed on 7 January 2013). []
NCT00567476 {published data only}
-
- NCT00567476. A Randomized, Open‐Label, Multicenter Study to Evaluate the Effect of Xolair (Omalizumab) as Add‐on Therapy to Inhaled Corticosteroid + Long‐Acting Beta Agonist in Fixed or Flexible Dosing Compared to Isolated Inhaled Corticosteroid + Long‐Acting Beta Agonist in Fixed or Flexible Dosing in the Asthma‐Related Quality of Life in Patients With Severe Persistent Allergic Asthma. www.clinicaltrials.gov/show/NCT00567476 (accessed 7 January 2013). []
NCT00624832 {unpublished data only}
-
- CIGE025A2210. A Randomized, Double‐Blind, Placebo‐Controlled Study to Demonstrate the Efficacy of Xolair in an Allergen Bronchoprovocation Study in Asthmatic Populations Defined by Serum IgE Concentrations. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 2 October 2010).
-
- NCT00624832. A Randomized, Double‐Blind, Placebo‐Controlled Study to Demonstrate the Efficacy of Xolair in an Allergen Bronchoprovocation Study in Asthmatic Populations Defined by Serum IgE Concentrations. www.clinicaltrials.gov/show/NCT00624832 (accessed 8 February 2013). []
NCT00639691 {published data only}
-
- NCT00639691. A Compassionate Access Protocol to Assess the Safety of XolairTM (Omalizumab)in Patients (≥ 6 Years Old) With Severe Allergic Asthma Who Remain Symptomatic Despite Optimal Therapy. www.clinicaltrials.gov/show/NCT00639691 (accessed 7 February 2013). []
NCT00777764 {published data only}
-
- NCT00777764. The Safety and Utility of Skin Testing With XOLAIR® (Omalizumab) and Placebo Omalizumab (Formulation Excipients). www.clinicaltrials.gov/show/NCT00777764 (accessed 7 February 2013). []
NCT00784485 {published data only}
-
- NCT00784485. Non‐Invasive Measures of Distal Lung Disease in Asthmatics Before and After Treatment With Omalizumab. www.clinicaltrials.gov/show/NCT00784485 (accessed 7 February 2013). []
NCT00829179 {published data only}
-
- NCT00829179. Role of RhuMab‐E25 in Reducing Exhaled NO in Allergic Asthma. www.clinicaltrials.gov/show/NCT00829179 (accessed 7 February 2013). []
NCT01155700 {published data only}
-
- NCT01155700. A 24 Week, Open Label, Multi‐center Evaluation of Pharmacokinetics and Pharmacodynamics, Efficacy and Safety of Omalizumab in Japanese Children (6 ‐ 15 Years) With Inadequately Controlled Allergic Asthma Despite Current Recommended Treatment. www.clinicaltrials.gov/show/NCT01155700 (accessed 7 February 2013). []
NCT01219036 {published data only}
-
- NCT01219036. The Use of Fractional Exhaled Nitric Oxide (FeNO) and Induced Sputum in the Identification of Non‐adherence in Difficult to Control Asthma. www.clinicaltrials.gov/show/NCT01219036 (accessed 7 February 2013). []
Nopp 2010 {published data only}
-
- Nopp A, Johansson SG, Adedoyin J, Ankerst J, Palmqvist M, Oman H, et al. After 6 years with Xolair; a 3‐year withdrawal follow‐up. Allergy 2010;65(1):56‐60. - PubMed
Oh 2010 {published data only}
-
- Oh C, Parker J, Geba G, Molfino N. Safety profile and clinical activity of multiple subcutaneous (SC) doses of MEDI‐528, a humanized anti‐interleukin‐9 monoclonal antibody, in subjects with asthma. European Respiratory Society Annual Congress; Sep 18‐22; Barcelona. 2010:[377].
Oh 2012 {published data only}
-
- Oh CK, McLaurin KK, Kim K, Hultquist M, Molfino NA. A phase 2b, randomized study to evaluate the clinical activity and safety profile of subcutaneous MEDI‐528, an anti‐IL‐9 monoclonal antibody, In adults with uncontrolled asthma [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185(Meeting Abstracts):A2760. []
Ong 2005 {published data only}
-
- Ong YE, Menzies‐Gow A, Barkans J, Benyahia F, Ou TT, Ying S, et al. Anti‐IgE (omalizumab) inhibits late‐phase reactions and inflammatory cells after repeat skin allergen challenge. Journal of Allergy and Clinical Immunology 2005;116(3):558‐64. - PubMed
Parker 2010 {published data only}
-
- Parker J, Brazinsky S, Miller DS, Nayak A, Korenblat PE, Sari S, et al. Randomized, double‐blind, placebo‐controlled, multicenter phase 2A study to evaluate the effect of a humanized interleukin‐9 monoclonal antibody (MEDI‐528) on exercise‐induced bronchospasm [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A5394.
Parker 2011 {published data only}
-
- Parker J, Wolansky LJ, Khatry D, Geba GP, Molfino NA. Brain magnetic resonance imaging in adults with asthma. Contemporary Clinical Trials 2011;32(1):86‐9. - PubMed
Parker 2011a {published data only}
-
- Parker JM, Oh CK, LaForce C, Miller SD, Pearlman DS, Le C, et al. Safety profile and clinical activity of multiple subcutaneous doses of MEDI‐528, a humanized anti‐interleukin‐9 monoclonal antibody, in two randomized phase 2a studies in subjects with asthma. BMC Pulmonary Medicine 2011;11:14. - PMC - PubMed
Patel 2009 {published data only}
-
- Patel BM, Chiang DT, Clark JP, Romero FA, Casale TB. Effects of omalizumab (Xolair®) on airway hyperresponsiveness [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S263.
Pavord 2012 {published data only}
-
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM). Lancet. England, 2012; Vol. 380, issue 9842:651‐9. [] - PubMed
Piper 2011 {published data only}
-
- Piper E, Brightling C, Niven R, Oh C, Faggioni R, Poon K, et al. Phase 2 randomized, double‐blind, placebo‐controlled study of tralokinumab, an anti‐IL‐13 monoclonal antibody, in moderate to severe asthma. European Respiratory Society Annual Congress; Sep 24‐28; Amsterdam. 2011; Vol. 38, issue 55:608s [3425].
Piper 2013 {published data only}
Q2143G {unpublished data only}
-
- Israel E, Cohn J, Meltzer E, McCarty J, Zheng B, Carroll A. Omalizumab does not induce thrombocytopenia in treatment of asthma [Abstract]. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):S146.
-
- Kaiser J. Medical Officer's Efficacy Review: BLA/STN 103976/0. www.fda.gov (accessed 7 February 2013):123‐36.
Riviere 2008 {published data only}
-
- Riviere GJ, Kuebler P, Jaffe JS, Yeh CM, Reynolds C, Brookman L. A liquid formulation of omalizumab is bioequivalent to the current lyophilized formulation [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A613.
Riviere 2009 {published data only}
-
- Riviere GJ, Abbi S, Koehne‐Voss S, Kim K, Jaffe JS. Bioequivalence of a new formulation of omalizumab, solution for injection in prefilled syringe, to the current lyophilized formulation [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E1873].
Riviere 2011 {published data only}
-
- Riviere GJ, Yeh CM, Reynolds CV, Brookman L, Kaiser G. Bioequivalence of a novel omalizumab solution for injection compared with the standard lyophilized powder formulation. Journal of Bioequivalence and Bioavailability 2011;3(6):144‐50.
Rubin 2012 {published data only}
-
- Rubin AS, Souza‐Machado A, Andradre‐Lima M, Ferreira F, Honda A, Matozo TM. Effect of omalizumab as add‐on therapy on asthma‐related quality of life in severe allergic asthma: a Brazilian study (QUALITX). Journal of Asthma 2012;49(3):288‐93. - PubMed
Scheerens 2011 {published data only}
-
- Scheerens H, Arron JR, Su Z, Zheng Y, Putnam W, Erickson RW, et al. Predictive and pharmacodynamic biomarkers of interleukin‐13 blockade: effect of lebrikizumab on late phase asthmatic response to allergen challenge [Abstract]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB164.
Stallings 2009 {published data only}
-
- Stallings A, McLaughlin A, Murphy D, Carper H, Platts‐Mills T, Heymann P. A surveillance study of natural rhinovirus colds in young adults with mild asthma [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S56.
Tajiri 2013 {published data only}
-
- Tajiri T, Matsumoto H, Hiraumi H, Ikeda H, Morita K, Izuhara K, et al. Efficacy of omalizumab in eosinophilic chronic rhinosinusitis. Annals of Allergy Asthma & Immunology 2013;110(5):387‐8. [] - PubMed
Townley 2011 {published data only}
-
- Townley R, Jourdheuil D, Jourdheuil F, DeMeyere‐Coursey K, Mahon L, Romero T, et al. The effects of omalizumab on bronchial and alveolar airway inflammation as measured by exhaled nitric oxide (ENO) in moderate to severe asthmatics [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A4478.
Wenzel 2013 {published data only}
-
- Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. New England Journal of Medicine 2013:Epub ahead of print. [] - PubMed
Wilson 2008 {published data only}
-
- Wilson SJ, Ward JA, Jarjoiur NN, Kraft M, Chung KF, Fahy JV, et al. Omalizumab reduces mast cell numbers in airway smooth muscle [Abstract]. American Thoracic Society International Conference; May 16‐21; Toronto. 2008:A499.
Yalcin 2011 {published data only}
-
- Yalcin AD, Kargi A, Kose S, Terzioglu E, Gorczynski RM. Efficacy of omalizumab and specific subcutaneous immunotherapy in allergic asthma. Respirology 2011;16(Suppl 2):Abstracts (pages 1‐326).
Zaidi 2009 {published data only}
-
- Zaidi AK, Saini SS, MacGlashan DW Jr. Changes in the Fc‐epsilonRI‐beta: Fc‐epsilonRI‐alpha ratio during treatment with omalizumab [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S193.
Zielen 2009 {published data only}
-
- Zielen S, Lieb A, Monchy J, Motte S, Wagner F, Fuhr R, et al. Omalizumab protects against allergen‐induced bronchoconstriction in patients with allergic (IgE‐mediated) asthma and high baseline IgE levels [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[E1869].
Zielen 2013 {published data only}
-
- Zielen S, Lieb A, Motte S, Wagner F, Monchy J, Fuhr R, et al. Omalizumab protects against allergen‐induced bronchoconstriction in allergic (immunoglobulin E‐‐mediated) asthma. International Archives of Allergy and Immunology 2013;160(1):102‐10. [] - PubMed
References to studies awaiting assessment
Creticos 2010 {published data only}
-
- Creticos PS, Saini SS, Scarupa MD, Balcer‐Whaley SL, Bieneman AP, Schroeder JT. Effects of omalizumab in non‐allergic asthma [Abstract]. Journal of Allergy and Clinical Immunology 2010;125(2 Suppl 1):AB197.
-
- NCT00162773. Effect of Anti‐IgE in Non‐Allergic Asthma. www.clinicaltrials.gov/show/NCT00162773 (accessed 7 February 2013). []
NCT00046748 {published data only}
-
- CIGE025A2306 28‐Wk, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study to Assess Efficacy, Safety, Tolerability of Subcutaneous Omalizumab in Adults and Adolescents With Severe Persistent Allergic Asthma Who Remain Inadequately Controlled Despite GINA (2002) Step 4 Therapy. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 February 2013).
-
- NCT00046748. Ph III, 28‐Wk, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group Study to Assess Efficacy, Safety, Tolerability of SC Omalizumab in Adults and Adolescents w/ Severe Persist. Allergic Asthma & Are Inadequately Controlled Despite GINA (2002) Step 4 Tx. www.clinicaltrials.gov/show/NCT00046748 (accessed 7 February 2013). []
NCT00226200 {published data only}
-
- NCT00226200. Soluble CD23 Expression as a Marker of Immunomodulation and Clinical Response in Asthma Patients Treated With Omalizumab. www.clinicaltrials.gov/show/NCT00226200 (accessed 7 February 2013). []
NCT00329381 {published data only}
-
- NCT00329381. A 26‐Wk, Randomized, Double‐Blinded,Parallel‐Group, Placebo‐Controlled,Multi‐Centered Study to Evaluate the Effect of Xolair (Omalizumab) on Improving the Tolerability of Spec. Immunotherapy in Patients With at Least Mod. Persistent Allergic Asthma Inadequately Controlled with Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00329381 (accessed 7 February 2013). []
NCT00367016 {published data only}
-
- NCT00367016. Immunologic Basis of Anti‐IgE Therapy (Study II: On Patients With Asthma). www.clinicaltrials.gov/show/NCT00367016 (accessed 7 February 2013). []
NCT00495612 {published data only}
-
- NCT00495612. A Study of Omalizumab in Preventing Bronchoconstriction Following Environmental Cat Dander Exposure in Patients With Cat Dander‐Induced Asthma (AERO). www.clinicaltrials.gov/ct2/show/NCT00495612 (accessed 7 February 2013).
NCT00670930 {published data only}
-
- CIGE025A2432 Efficacy of Omalizumab in Adults (18‐60 Years of Age) With Moderate‐Severe, Persistent Allergic Asthma, Despite Receiving Inhaled Corticosteroids and Long Acting Beta‐Agonists. http://www.novctrd.com/ctrdWebApp/clinicaltrialrepository/displayFile.do... (accessed 7 February 2013).
-
- NCT00670930. A Randomized, Multi‐Center, Double‐Blind, Placebo‐Controlled, Parallel‐Group Trial to Explore the Effects of 78 Weeks Omalizumab Treatment Given as Add on Therapy on Markers of Airway Inflammation and Remodeling in Patients With Moderate to Severe Persistent Allergic Asthma Receiving Inhaled Corticosteroids and Long Acting Beta‐agonists. www.clinicaltrials.gov/show/NCT00670930 (accessed 7 February 2013). []
NCT00691873 {published data only}
-
- NCT00691873. A 26‐Wk, Randomized, Dble‐Blinded, Parallel‐Grp, Placebo‐Controlled, Multi‐Centered Study to Eval the Effect of Xolair (Omalizumab) on Improving the Tolerability of Spec. Immunotherapy in Patients With at Least Mod. Persistent Allergic Asthma Inadequately Controlled w/Inhaled Corticosteroids. www.clinicaltrials.gov/show/NCT00691873 (accessed 7 February 2013). []
NCT01393340 {published data only}
-
- NCT01393340. Clinical and Biological Effects of Anti‐IgE (Omalizumab) in Patients With Bilateral Nasal Polyposis and Asthma. www.clinicaltrials.gov/show/NCT01393340 (accessed 7 February 2013). []
Scripps 2009 {published data only}
-
- NCT00286416. Double Blind Study to Determine Effect of Omalizumab Treatment in Patients With the Co‐Morbid Conditions of Aspirin Exacerbated Respiratory Disease(AERD) and Allergic Asthma and Rhinitis. www.clinicaltrials.gov/show/NCT00286416 (accessed 7 February 2013). []
-
- Scripps Clinic. Double Blind Study to Determine Effect of Omalizumab Treatment in Patients With the Co‐morbid Conditions of Aspirin Exacerbated Respiratory Disease (AERD) and Allergic Asthma and Rhinitis (completed). NCT00286416 2009:ClinicalTrials.gov ID: NCT00286416.
References to ongoing studies
NCT00139152 {published data only}
-
- NCT00139152. Exhaled Breath Condensate and Nitric Oxide: Non‐Invasive Evaluation of Lung Disease After Treatment With Xolair. www.clinicaltrials.gov/show/NCT00139152 (accessed 7 February 2013). []
NCT00208234 {published data only}
-
- NCT00208234. The Effects of Xolair (Omalizumab) on Airway Hyperresponsiveness. www.clinicaltrials.gov/show/NCT00208234 (accessed 7 February 2013). []
NCT00555971 {published data only}
-
- NCT00555971. A Placebo Controlled, Double‐Blind Investigation of the Therapeutic Utility of Xolair (Omalizumab) for Attenuating Aspirin Induced Bronchospasm in Patients With Aspirin Exacerbated Respiratory Disease (AERD) Undergoing Aspirin Desentization. www.clinicaltrials.gov/show/NCT00555971 (accessed 7 February 2013). []
NCT01113437 {published data only}
-
- NCT01113437. The Effect of a Humanised Monoclonal Anti‐IgE Antibody, Omalizumab, on Disease Control and Bronchial Mucosal Inflammation in Non‐Atopic Asthma. www.clinicaltrials.gov/show/NCT01113437 (accessed 7 February 2013). []
NCT01125748 {published data only}
-
- NCT01125748. A Phase IV, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Study Evaluating the Persistency of Response With or Without Xolair After Long‐Term Therapy. www.clinicaltrials.gov/show/NCT01125748 (accessed 7 February 2013). []
NCT01202903 {published data only}
-
- NCT01202903. A 24‐Week, Phase III Randomized, Double‐Blind, Placebo‐Controlled, Parallel‐Group, Multicenter Study of Xolair® (Omalizumab) in Patients With Moderate to Severe Persistent Allergic Asthma Who Remain Not Adequately Controlled Despite GINA (2009) Step 4 Therapy. www.clinicaltrials.gov/show/NCT01202903 (accessed 7 February 2013). []
NCT01430403 {published data only}
-
- NCT01430403. Preventative Omalizumab or Step‐Up Therapy for Severe Fall Exacerbations (ICAC‐20). www.clinicaltrials.gov/show/NCT01430403 (accessed 7 February 2013). []
NCT01544348 {published data only}
-
- NCT01544348. A Phase 1, Randomized, Placebo‐Controlled, Dose‐Escalation Study to Evaluate the Safety of MEDI4212 in Allergic Subjects. www.clinicaltrials.gov/show/NCT01544348 (accessed 7 February 2013). []
Additional references
Arshad 2001
-
- Arshad SH, Babu KS, Holgate ST. Anti‐IgE Therapy in Asthma and Allergy. 1st Edition. London: Martin Dunitz, 2001.
Bousquet 2004
-
- Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti‐IgE antibody, in patients with allergic asthma. Chest 2004;125(4):1378‐86. - PubMed
BTS 2005
-
- British Thoracic Society. British guideline on the management of asthma. www.sign.ac.uk/pdf/sign101.pdf (accessed 7 February 2013).
BTS/SIGN 2012
-
- British Guideline on the Management of Asthma. A National Clinical Guideline. www.sign.ac.uk/guidelines/fulltext/101/ (accessed 7 February 2013).
Burrows 1989
-
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE and skin test reactivity to allergens. New England Journal of Medicine 1989;320:271‐7. - PubMed
Casale 1997
-
- Casale TB, Bernstein IL, Busse WW, et al. Use of an anti‐IgE humanised monoclonal antibody in ragweed‐induced allergic rhinitis. Journal of Allergy and Clinical Immunology 1997;100:110‐21. - PubMed
Chen 2012
-
- Chen H, Eisner MD, Haselkorn T, Trzaskoma B. Concomitant asthma medications in moderate‐to‐severe allergic asthma treated with omalizumab. Respiratory Medicine 2012 October 18 [Epub ahead of print]. - PubMed
Chipps 2012
-
- Chipps BE, Figliomeni M, Spector S. Omalizumab: an update on efficacy and safety in moderate‐to‐severe allergic asthma. Allergy Asthma Proceedings 2012;33(5):377‐85. - PubMed
CTS 2012
-
- 2012 CTS Guideline Update: diagnosis and management of asthma in preschoolers, children and adults. www.respiratoryguidelines.ca/2012‐cts‐guideline‐asthma‐update (accessed 7 February 2013).
GiNA 2011
-
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2011. www.ginasthma.org/guidelines‐gina‐report‐global‐strategy‐for‐asthma.html (accessed 16 September 2012).
Haldar 2008
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jadad 1996
-
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Juniper 1994
-
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47:81‐7. - PubMed
Milgrom 1997
-
- Milgrom H, Fick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti‐IgE antibody. New England Journal of Medicine 1999;341(26):1966‐73. - PubMed
NICE 2013
-
- National Institute for Health and Care Excellence. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6 and over and adults (review of TA133 and TA201). http://www.nice.org.uk/nicemedia/live/14157/63689/63689.pdf (accessed 25 June 2013).
Pelaia 2011
Perret 2013
-
- Perret JL, Dharmage SC, Matheson MC, Johns DP, Gurrin LC, et al. The interplay between the effects of lifetime asthma, smoking, and atopy on fixed airflow obstruction in middle age. American Journal of Respiratory and Critical Care Medicine 2013;187(1):42‐8. - PubMed
Qureshi 1998
-
- Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. New England Journal of Medicine 1998;339:1030‐5. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sears 1991
-
- Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relationship between airways responsiveness and serum IgE in children with asthma and in apparently normal children. New England Journal of Medicine 1991;325:1067‐71. - PubMed
Spector 1999
-
- Spector SL. Allergic inflammation in upper and lower airways. Annals of Allergy Asthma & Immunology 1999;83:435‐44. - PubMed
Thomson 2012
Walker 2011
-
- Walker S. Omalizumab reduces frequency of asthma exacerbations in children. Journal of Pediatrics 2011;159(3):512‐3. - PubMed
Wenzel 2002
-
- Wenzel S, Bousquet J, Holgate S, Freeman P, Fox H. Patients with more severe allergic asthma gain greatest relative benefit from omalizumab therapy. American Journal of Respiratory & Critical Care Medicine. 2002; Vol. 165:A215.
Wijnhoven 2001
-
- Wijnhoven HAH, Kriegsman DMW, Hesselink AE, Penninx BWJH, Haan M. Determinants of different dimensions of disease severity in asthma and COPD: pulmonary function and quality of life. Chest 2001;119(4):1034‐42. - PubMed
Wills‐Karp 1999
-
- Wills‐Karp M. Immunologic basis of antigen‐induced airway hyperresponsiveness. Annual Review of Immunology 1999;17:255‐81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

