Differentiation therapy for the treatment of t(8;21) acute myeloid leukemia using histone deacetylase inhibitors
- PMID: 24415537
- PMCID: PMC3938147
- DOI: 10.1182/blood-2013-03-488114
Differentiation therapy for the treatment of t(8;21) acute myeloid leukemia using histone deacetylase inhibitors
Abstract
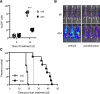
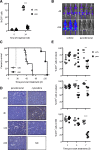
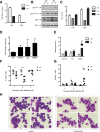
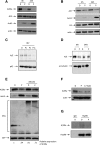
Epigenetic modifying enzymes such as histone deacetylases (HDACs), p300, and PRMT1 are recruited by AML1/ETO, the pathogenic protein for t(8;21) acute myeloid leukemia (AML), providing a strong molecular rationale for targeting these enzymes to treat this disease. Although early phase clinical assessment indicated that treatment with HDAC inhibitors (HDACis) may be effective in t(8;21) AML patients, rigorous preclinical studies to identify the molecular and biological events that may determine therapeutic responses have not been performed. Using an AML mouse model driven by expression of AML1/ETO9a (A/E9a), we demonstrated that treatment of mice bearing t(8;21) AML with the HDACi panobinostat caused a robust antileukemic response that did not require functional p53 nor activation of conventional apoptotic pathways. Panobinostat triggered terminal myeloid differentiation via proteasomal degradation of A/E9a. Importantly, conditional A/E9a deletion phenocopied the effects of panobinostat and other HDACis, indicating that destabilization of A/E9a is critical for the antileukemic activity of these agents.
Figures



References
-
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. - PubMed
-
- Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. - PubMed
-
- Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

