Pregabalin monotherapy in patients with partial-onset seizures: a historical-controlled trial
- PMID: 24415567
- PMCID: PMC3963419
- DOI: 10.1212/WNL.0000000000000119
Pregabalin monotherapy in patients with partial-onset seizures: a historical-controlled trial
Abstract
Objective: To assess pregabalin monotherapy for partial-onset seizures using a historical-controlled conversion-to-monotherapy design.
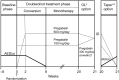
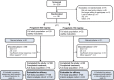
Methods: Adults with inadequately controlled partial-onset seizures while receiving 1 or 2 antiepileptic drugs during an 8-week prospective baseline were randomized to double-blind monotherapy with pregabalin 600 or 150 mg/d (4:1) for 20 weeks (8-week conversion and 12-week monotherapy period). The primary endpoint was the seizure-related exit rate for pregabalin 600 mg/d, based on discontinuations due to predefined criteria. Efficacy was declared if the upper limit of the 95% confidence interval for the exit rate was below a historical-control threshold of 74%, with stepwise evaluation using a threshold of 68%.
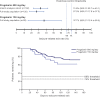
Results: The trial was stopped early for positive efficacy after an interim analysis in 125 patients. The full study population included 161 patients, with 148 evaluable for efficacy. The mean time since epilepsy diagnosis was 14 years. Overall, 54.3% (600 mg/d) and 46.9% (150 mg/d) of patients completed 20 weeks of double-blind treatment. Seizure-related exit rate in the 600 mg/d group (27.5%; 95% confidence interval, 17.8%-37.2%) was significantly below the 74% and 68% thresholds (p < 0.001 for both). Eight patients on 600 mg/d and 2 on 150 mg/d were seizure-free throughout pregabalin monotherapy. Pregabalin's overall safety profile was consistent with prior trials.
Conclusions: Pregabalin monotherapy was safe and efficacious for patients with inadequately controlled partial-onset seizures.
Classification of evidence: This study provides Class III evidence that patients with inadequately controlled partial-onset seizures switched to pregabalin monotherapy have fewer seizure-related exit events compared with historical controls switched to pseudo-placebo monotherapy.
Trial registration: ClinicalTrials.gov NCT00524030.
Figures
References
-
- French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology 2003;60:1631–1637 - PubMed
-
- Arroyo S, Anhut H, Kugler AR, et al. Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epilepsia 2004;45:20–27 - PubMed
-
- Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology 2005;64:475–480 - PubMed
-
- Kwan P, Brodie MJ, Kalviainen R, Yurkewicz L, Weaver J, Knapp LE. Efficacy and safety of pregabalin versus lamotrigine in patients with newly diagnosed partial seizures: a phase 3, double-blind, randomised, parallel-group trial. Lancet Neurol 2011;10:881–890 - PubMed
-
- Perucca E. Designing clinical trials to assess antiepileptic drugs as monotherapy: difficulties and solutions. CNS Drugs 2008;22:917–938 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
