Molecular specificity, convergence and constraint shape adaptive evolution in nutrient-poor environments
- PMID: 24415948
- PMCID: PMC3886903
- DOI: 10.1371/journal.pgen.1004041
Molecular specificity, convergence and constraint shape adaptive evolution in nutrient-poor environments
Abstract
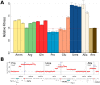
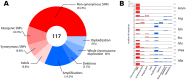
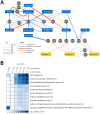
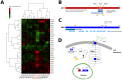
One of the central goals of evolutionary biology is to explain and predict the molecular basis of adaptive evolution. We studied the evolution of genetic networks in Saccharomyces cerevisiae (budding yeast) populations propagated for more than 200 generations in different nitrogen-limiting conditions. We find that rapid adaptive evolution in nitrogen-poor environments is dominated by the de novo generation and selection of copy number variants (CNVs), a large fraction of which contain genes encoding specific nitrogen transporters including PUT4, DUR3 and DAL4. The large fitness increases associated with these alleles limits the genetic heterogeneity of adapting populations even in environments with multiple nitrogen sources. Complete identification of acquired point mutations, in individual lineages and entire populations, identified heterogeneity at the level of genetic loci but common themes at the level of functional modules, including genes controlling phosphatidylinositol-3-phosphate metabolism and vacuole biogenesis. Adaptive strategies shared with other nutrient-limited environments point to selection of genetic variation in the TORC1 and Ras/PKA signaling pathways as a general mechanism underlying improved growth in nutrient-limited environments. Within a single population we observed the repeated independent selection of a multi-locus genotype, comprised of the functionally related genes GAT1, MEP2 and LST4. By studying the fitness of individual alleles, and their combination, as well as the evolutionary history of the evolving population, we find that the order in which these mutations are acquired is constrained by epistasis. The identification of repeatedly selected variation at functionally related loci that interact epistatically suggests that gene network polymorphisms (GNPs) may be a frequent outcome of adaptive evolution. Our results provide insight into the mechanistic basis by which cells adapt to nutrient-limited environments and suggest that knowledge of the selective environment and the regulatory mechanisms important for growth and survival in that environment greatly increase the predictability of adaptive evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Dean AM, Thornton JW (2007) Mechanistic approaches to the study of evolution: the functional synthesis. Nature Reviews Genetics 8: 675–688 doi:10.1038/nrg2160 - DOI - PMC - PubMed
-
- Thomson JM, Gaucher EA, Burgan MF, De Kee DW, Li T, et al. (2005) Resurrecting ancestral alcohol dehydrogenases from yeast. Nature genetics 37: 630–635 doi:10.1038/ng1553 - DOI - PMC - PubMed
-
- Bridgham JT, Ortlund EA, Thornton JW (2009) An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461: 515–519 doi:10.1038/nature08249 - DOI - PMC - PubMed
-
- Elena SF, Lenski RE (2003) Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nature Reviews Genetics 4: 457–469 doi:10.1038/nrg1088 - DOI - PubMed
-
- Novick A, Szilard L (1950) Description of the chemostat. Science 112: 715–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

