Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications
- PMID: 24415978
- PMCID: PMC3808214
- DOI: 10.1159/000354253
Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications
Abstract
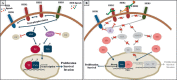
The estrogen receptor (ER) and/or the human epidermal growth factor receptor 2 (HER2) signaling pathways are the dominant drivers of cell proliferation and survival in the majority of human breast cancers. As a result, targeting these pathways provides the most effective therapies in appropriately selected patients. Nevertheless, resistance to both endocrine and anti-HER2 therapies occurs frequently and represents a major clinical challenge. Compelling preclinical and clinical evidence relates this treatment resistance to the presence of a complex bidirectional molecular crosstalk between the ER and HER2 pathways. As a consequence, treatment strategies targeting either pathway are associated with up-regulation of the other one, ultimately resulting in resistance to therapy. Therefore, a more promising strategy to prevent or overcome either endocrine or anti-HER2 resistance at least in some tumors is to combine targeted treatments that simultaneously block both signaling pathways. Many clinical trials exploring this strategy have shown positive results, and many more are currently ongoing. Future clinical trials with appropriate patient selection, based on biomarker evaluation of primary tumors and possibly of recurrent lesions, are warranted for the optimization of individualized therapeutic strategies.
Die Signalwege des Östrogenrezeptors (ER) und/oder des humanen epidermalen Wachstumsfaktorrezeptors 2 (HER2) sind bei der Mehrzahl der Brustkrebsarten des Menschen die ausschlaggebenden Faktoren für die Proliferation und das Überleben von Zellen. Eine Blockade dieser Signalwege ermöglicht deshalb die wirksamsten Therapien bei entsprechend ausgewählten Patientinnen. Dennoch kommt es oft zu einer Resistenz sowohl gegen endokrine als gegen auch Anti-HER2-Therapien, die in der klinischen Praxis eine große Herausforderung darstellt. Es gibt überzeugende präklinische und klinische Hinweise, dass diese Behandlungsresistenz mit der Existenz einer komplexen gegenseitigen Beeinflussung zwischen den ER- und HER2-Signalwegen zusammenhängt. Aus diesem Grund sind Behandlungsstrategien, die auf einen der Signalwege abzielen, mit der Hochregulation des anderen Signalwegs verknüpft, was letztendlich zur Therapieresistenz führt. Eine aussichtsreichere Strategie, um eine endokrine oder Anti-HER2-Resistenz zumindest bei einigen Tumoren zu verhindern oder zu überwinden, ist deshalb die Kombination von gezielten Therapien, die beide Signalwege gleichzeitig hemmen. Viele klinische Studien, die diese Strategie erforschen, haben positive Resultate gezeigt und viele weitere sind zurzeit noch nicht abgeschlossen. Zukünftige klinische Studien, die auf einer Evaluation der Primärtumoren und eventuell erneut auftretender Tumoren mithilfe von Biomarkern beruhen, sollten zur Optimierung von individualisierten Therapiestrategien durchgeführt werden.
Keywords: Crosstalk; Estrogen Receptor; HER2; Resistance.
Figures

References
-
- Forouzanfar MH, Foreman KJ, Delossantos AM, Lozano R, Lopez AD, Murray CJ, Naghavi M. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. - PubMed
-
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. - PMC - PubMed
-
- Osborne CK, Schiff R, Fuqua SA, Shou J. Estrogen receptor: current understanding of its activation and modulation. Clin Cancer Res. 2001;7:4338s–4342s. discussion 4411s–4412s. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

