A simple approach for synthesis, characterization and bioactivity of bovine bones to fabricate the polyurethane nanofiber containing hydroxyapatite nanoparticles
- PMID: 24416082
- PMCID: PMC3886683
- DOI: 10.3144/expresspolymlett.2012.5
A simple approach for synthesis, characterization and bioactivity of bovine bones to fabricate the polyurethane nanofiber containing hydroxyapatite nanoparticles
Abstract
In the present study, we had introduced polyurethane (PU) nanofibers that contain hydroxyapatite (HAp) nanoparticles (NPs) as a result of an electrospinning process. A simple method that does not depend on additional foreign chemicals had been employed to synthesize HAp NPs through the calcination of bovine bones. Typically, a colloidal gel consisting of HAp/PU had been electrospun to form nanofibers. In this communication, physiochemical aspects of prepared nanofibers were characterized by FE-SEM, TEM and TEM-EDS, which confirmed that nanofibers were well-oriented and good dispersion of HAp NPs, over the prepared nanofibers. Parameters, affecting the utilization of the prepared nanofibers in various nano-biotechnological fields have been studied; for instance, the bioactivity of the produced nanofiber mats was investigated while incubating in simulated body fluid (SBF). The results from incubation of nanofibers, indicated that incorporation of HAp strongly activates the precipitation of the apatite-like particles, because of the HAp NPs act as seed, that accelerate crystallization of the biological HAp from the utilized SBF.
Keywords: biocompatible polymers; nanomaterials.
Figures
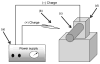

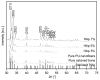
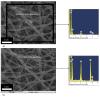


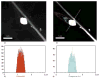
 in
in
 (c) and
(c) and
 in
in
 (d).
(d).




References
-
- LeGeros RZ. Calcium phosphates in oral biology and medicine. Karger; Basel: 1991. - PubMed
-
- Lü XY, Fan YB, Gu D, Cui W. Preparation and characterization of natural hydroxyapatite from animal hard tissues. Key Engineering Materials. 2007;342–343:213–342. DOI: 10.4028/ www.scientific.net/KEM.342-343.213.
-
- Ozawa M, Suzuki S. Microstructural development of natural hydroxyapatite originated from fish-bone waste through heat treatment. Journal of the American Ceramic Society. 2002;85:1315–1317. doi: 10.1111/j.1151-2916.2002.tb00268.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
