Photoacoustic Microscopy
- PMID: 24416085
- PMCID: PMC3887369
- DOI: 10.1002/lpor.201200060
Photoacoustic Microscopy
Abstract
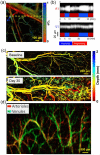
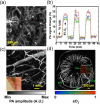
Photoacoustic microscopy (PAM) is a hybrid in vivo imaging technique that acoustically detects optical contrast via the photoacoustic effect. Unlike pure optical microscopic techniques, PAM takes advantage of the weak acoustic scattering in tissue and thus breaks through the optical diffusion limit (~1 mm in soft tissue). With its excellent scalability, PAM can provide high-resolution images at desired maximum imaging depths up to a few millimeters. Compared with backscattering-based confocal microscopy and optical coherence tomography, PAM provides absorption contrast instead of scattering contrast. Furthermore, PAM can image more molecules, endogenous or exogenous, at their absorbing wavelengths than fluorescence-based methods, such as wide-field, confocal, and multi-photon microscopy. Most importantly, PAM can simultaneously image anatomical, functional, molecular, flow dynamic and metabolic contrasts in vivo. Focusing on state-of-the-art developments in PAM, this Review discusses the key features of PAM implementations and their applications in biomedical studies.
Keywords: Photoacoustic microscopy; blood flow imaging; brain imaging; functional imaging; gene expression imaging; metabolic imaging; molecular imaging; multi-scale imaging; nanoparticle imaging; real-time imaging; tumor imaging.
Figures
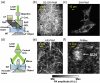
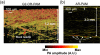

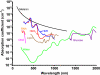
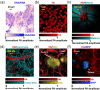


References
-
- Amos B. Lessons from the history of light microscopy. Nature Cell Biology. 2000;2:E151–E152. - PubMed
-
- Wang LV, Wu H.-i. Biomedical optics : principles and imaging. xiv. Wiley-Interscience; Hoboken, N.J.: 2007. p. 362.
-
- Wang LV. Tutorial on photoacoustic microscopy and computed tomography. IEEE Journal of Selected Topics in Quantum Electronics. 2008;14:171–179.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
