Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium
- PMID: 24416126
- PMCID: PMC3885380
- DOI: 10.1371/journal.pone.0080050
Anti-tumoral effect of the mitochondrial target domain of Noxa delivered by an engineered Salmonella typhimurium
Abstract
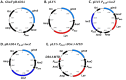
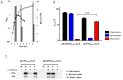
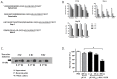
Bacterial cancer therapy relies on the fact that several bacterial species are capable of targeting tumor tissue and that bacteria can be genetically engineered to selectively deliver therapeutic proteins of interest to the targeted tumors. However, the challenge of bacterial cancer therapy is the release of the therapeutic proteins from the bacteria and entry of the proteins into tumor cells. This study employed an attenuated Salmonella typhimurium to selectively deliver the mitochondrial targeting domain of Noxa (MTD) as a potential therapeutic cargo protein, and examined its anti-cancer effect. To release MTD from the bacteria, a novel bacterial lysis system of phage origin was deployed. To facilitate the entry of MTD into the tumor cells, the MTD was fused to DS4.3, a novel cell-penetrating peptide (CPP) derived from a voltage-gated potassium channel (Kv2.1). The gene encoding DS4.3-MTD and the phage lysis genes were placed under the control of PBAD , a promoter activated by L-arabinose. We demonstrated that DS4.3-MTD chimeric molecules expressed by the Salmonellae were anti-tumoral in cultured tumor cells and in mice with CT26 colon carcinoma.
Conflict of interest statement
Figures


Similar articles
-
Targeted deletion of the ara operon of Salmonella typhimurium enhances L-arabinose accumulation and drives PBAD-promoted expression of anti-cancer toxins and imaging agents.Cell Cycle. 2014;13(19):3112-20. doi: 10.4161/15384101.2014.949527. Cell Cycle. 2014. PMID: 25486570 Free PMC article.
-
Minimal killing unit of the mitochondrial targeting domain of Noxa.J Pept Sci. 2013 Aug;19(8):485-90. doi: 10.1002/psc.2525. Epub 2013 Jun 22. J Pept Sci. 2013. PMID: 23794461
-
Designing a cancer therapeutic peptide by combining the mitochondrial targeting domain of Noxa and ErbB2-targeting moieties.FEBS Lett. 2018 Jan;592(1):103-111. doi: 10.1002/1873-3468.12922. Epub 2017 Dec 11. FEBS Lett. 2018. PMID: 29193033
-
Tumor-targeting amino acid auxotrophic Salmonella typhimurium.Amino Acids. 2009 Sep;37(3):509-21. doi: 10.1007/s00726-009-0261-8. Epub 2009 Mar 17. Amino Acids. 2009. PMID: 19291366 Review.
-
Genetically engineered Salmonella Typhimurium: Recent advances in cancer therapy.Cancer Lett. 2019 Apr 28;448:168-181. doi: 10.1016/j.canlet.2019.01.037. Epub 2019 Feb 10. Cancer Lett. 2019. PMID: 30753837 Review.
Cited by
-
Engineering the gut microbiota to treat chronic diseases.Appl Microbiol Biotechnol. 2020 Sep;104(18):7657-7671. doi: 10.1007/s00253-020-10771-0. Epub 2020 Jul 21. Appl Microbiol Biotechnol. 2020. PMID: 32696297 Free PMC article. Review.
-
Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents.Molecules. 2018 Jan 31;23(2):295. doi: 10.3390/molecules23020295. Molecules. 2018. PMID: 29385037 Free PMC article. Review.
-
Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium.Mol Ther. 2022 Feb 2;30(2):662-671. doi: 10.1016/j.ymthe.2021.08.023. Epub 2021 Aug 14. Mol Ther. 2022. PMID: 34400328 Free PMC article.
-
In Silico Selection and Evaluation of Pugnins with Antibacterial and Anticancer Activity Using Skin Transcriptome of Treefrog (Boana pugnax).Pharmaceutics. 2021 Apr 18;13(4):578. doi: 10.3390/pharmaceutics13040578. Pharmaceutics. 2021. PMID: 33919639 Free PMC article.
-
Targeted Cancer Therapy Using Engineered Salmonella typhimurium.Chonnam Med J. 2016 Sep;52(3):173-84. doi: 10.4068/cmj.2016.52.3.173. Epub 2016 Sep 23. Chonnam Med J. 2016. PMID: 27689027 Free PMC article. Review.
References
-
- Levesque AA, Eastman A (2007) p53-based cancer therapies: Is defective p53 the Achilles heel of the tumor? Carcinogenesis 28: 13–20. - PubMed
-
- Martins CP, Brown-Swigart L, Evan GI (2006) Modeling the therapeutic efficacy of p53 restoration in tumors. Cell 127: 1323–1334. - PubMed
-
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770–776. - PubMed
-
- Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22: 8590–8607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

