Targeting a cross-reactive Gly m 5 soy peptide as responsible for hypersensitivity reactions in a milk allergy mouse model
- PMID: 24416141
- PMCID: PMC3886974
- DOI: 10.1371/journal.pone.0082341
Targeting a cross-reactive Gly m 5 soy peptide as responsible for hypersensitivity reactions in a milk allergy mouse model
Abstract
Background: Cross-reactivity between soybean allergens and bovine caseins has been previously reported. In this study we aimed to map epitopes of the major soybean allergen Gly m 5 that are co-recognized by casein specific antibodies, and to identify a peptide responsible for the cross-reactivity.
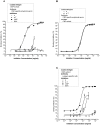
Methods: Cow's milk protein (CMP)-specific antibodies were used in different immunoassays (immunoblotting, ELISA, ELISA inhibition test) to evaluate the in vitro recognition of soybean proteins (SP). Recombinant Gly m 5 (α), a truncated fragment containing the C-terminal domain (α-T) and peptides of α-T were obtained and epitope mapping was performed with an overlapping peptide assay. Bioinformatics tools were used for epitope prediction by sequence alignment, and for modelling the cross-recognized soy proteins and peptides. The binding of SP to a monoclonal antibody was studied by surface Plasmon resonance (SPR). Finally, the in vivo cross-recognition of SP was assessed in a mouse model of milk allergy.
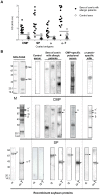
Results: Both α and α-T reacted with the different CMP-specific antibodies. α-T contains IgG and IgE epitopes in several peptides, particularly in the peptide named PA. Besides, we found similar values of association and dissociation constants between the α-casein specific mAb and the different milk and soy components. The food allergy mouse model showed that SP and PA contain the cross-reactive B and T epitopes, which triggered hypersensitivity reactions and a Th2-mediated response on CMP-sensitized mice.
Conclusions: Gly m 5 is a cross-reactive soy allergen and the α-T portion of the molecule contains IgG and IgE immunodominant epitopes, confined to PA, a region with enough conformation to be bound by antibodies. These findings contribute to explain the intolerance to SP observed in IgE-mediated CMA patients, primarily not sensitised to SP, as well as it sets the basis to propose a mucosal immunotherapy for milk allergy using this soy peptide.
Conflict of interest statement
Figures




Similar articles
-
Immunochemical characterization of Glycine max L. Merr. var Raiden, as a possible hypoallergenic substitute for cow's milk-allergic patients.Clin Exp Allergy. 2008 Sep;38(9):1559-65. doi: 10.1111/j.1365-2222.2008.03062.x. Epub 2008 Jul 9. Clin Exp Allergy. 2008. PMID: 18631353
-
Immunoglobulin E and immunoglobulin G cross-reactive allergens and epitopes between cow milk αS1-casein and soybean proteins.J Dairy Sci. 2020 Nov;103(11):9815-9824. doi: 10.3168/jds.2020-18250. Epub 2020 Sep 28. J Dairy Sci. 2020. PMID: 32896409
-
A novel approach to ameliorate experimental milk allergy based on the oral administration of a short soy cross-reactive peptide.Food Chem. 2021 Jun 1;346:128926. doi: 10.1016/j.foodchem.2020.128926. Epub 2020 Dec 26. Food Chem. 2021. PMID: 33484948
-
Milk and soy allergy.Pediatr Clin North Am. 2011 Apr;58(2):407-26, x. doi: 10.1016/j.pcl.2011.02.005. Pediatr Clin North Am. 2011. PMID: 21453810 Free PMC article. Review.
-
Cow's milk allergy: where have we come from and where are we going?Endocr Metab Immune Disord Drug Targets. 2014 Mar;14(1):2-8. doi: 10.2174/1871530314666140121142900. Endocr Metab Immune Disord Drug Targets. 2014. PMID: 24450456 Review.
Cited by
-
Overview of in vivo and ex vivo endpoints in murine food allergy models: Suitable for evaluation of the sensitizing capacity of novel proteins?Allergy. 2020 Feb;75(2):289-301. doi: 10.1111/all.13943. Epub 2019 Jul 9. Allergy. 2020. PMID: 31187876 Free PMC article. Review.
-
Unlocking the Secrets of Human Milk: Isolation and Characterization of Extracellular Vesicles.Adv Nutr. 2025 Jun;16(6):100430. doi: 10.1016/j.advnut.2025.100430. Epub 2025 Apr 25. Adv Nutr. 2025. PMID: 40288493 Free PMC article. Review.
-
Orally-Induced Intestinal CD4+ CD25+ FoxP3+ Treg Controlled Undesired Responses towards Oral Antigens and Effectively Dampened Food Allergic Reactions.PLoS One. 2015 Oct 30;10(10):e0141116. doi: 10.1371/journal.pone.0141116. eCollection 2015. PLoS One. 2015. PMID: 26517875 Free PMC article.
-
Improving serodiagnosis of human and canine leishmaniasis with recombinant Leishmania braziliensis cathepsin l-like protein and a synthetic peptide containing its linear B-cell epitope.PLoS Negl Trop Dis. 2015 Jan 8;9(1):e3426. doi: 10.1371/journal.pntd.0003426. eCollection 2015 Jan. PLoS Negl Trop Dis. 2015. PMID: 25569432 Free PMC article.
-
Cross-reactivity between the soybean protein p34 and bovine caseins.Allergy Asthma Immunol Res. 2015 Jan;7(1):60-8. doi: 10.4168/aair.2015.7.1.60. Epub 2014 Sep 11. Allergy Asthma Immunol Res. 2015. PMID: 25553264 Free PMC article.
References
-
- Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, et al. (2007) The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol 120: 638–646 doi:10.1016/j.jaci.2007.05.026 - DOI - PubMed
-
- Sicherer SH (2011) Food allergy. Mt Sinai J Med New York 78: 683–696 doi:10.1002/msj.20292 - DOI - PubMed
-
- Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, et al. (2010) World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. World Allergy Organ J 3: 57–161 doi:10.1097/WOX.0b013e3181defeb9 - DOI - PMC - PubMed
-
- Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, et al. (2012) Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 55: 221–229 doi:10.1097/MPG.0b013e31825c9482 - DOI - PubMed
-
- Dupont C (2011) Food allergy: recent advances in pathophysiology and diagnosis. Ann Nutr Metab 59 Suppl 1: 8–18 doi:10.1159/000334145 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

