Diffuse brain injury induces acute post-traumatic sleep
- PMID: 24416145
- PMCID: PMC3885381
- DOI: 10.1371/journal.pone.0082507
Diffuse brain injury induces acute post-traumatic sleep
Abstract
Objective: Clinical observations report excessive sleepiness immediately following traumatic brain injury (TBI); however, there is a lack of experimental evidence to support or refute the benefit of sleep following a brain injury. The aim of this study is to investigate acute post-traumatic sleep.
Methods: Sham, mild or moderate diffuse TBI was induced by midline fluid percussion injury (mFPI) in male C57BL/6J mice at 9:00 or 21:00 to evaluate injury-induced sleep behavior at sleep and wake onset, respectively. Sleep profiles were measured post-injury using a non-invasive, piezoelectric cage system. In separate cohorts of mice, inflammatory cytokines in the neocortex were quantified by immunoassay, and microglial activation was visualized by immunohistochemistry.
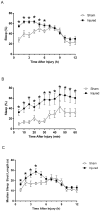
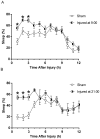
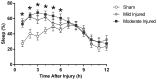
Results: Immediately after diffuse TBI, quantitative measures of sleep were characterized by a significant increase in sleep (>50%) for the first 6 hours post-injury, resulting from increases in sleep bout length, compared to sham. Acute post-traumatic sleep increased significantly independent of injury severity and time of injury (9:00 vs 21:00). The pro-inflammatory cytokine IL-1β increased in brain-injured mice compared to sham over the first 9 hours post-injury. Iba-1 positive microglia were evident in brain-injured cortex at 6 hours post-injury.
Conclusion: Post-traumatic sleep occurs for up to 6 hours after diffuse brain injury in the mouse regardless of injury severity or time of day. The temporal profile of secondary injury cascades may be driving the significant increase in post-traumatic sleep and contribute to the natural course of recovery through cellular repair.
Conflict of interest statement
Figures


Similar articles
-
Resolvins AT-D1 and E1 differentially impact functional outcome, post-traumatic sleep, and microglial activation following diffuse brain injury in the mouse.Brain Behav Immun. 2015 Jul;47:131-40. doi: 10.1016/j.bbi.2015.01.001. Epub 2015 Jan 10. Brain Behav Immun. 2015. PMID: 25585137 Free PMC article.
-
Diffuse brain injury does not affect chronic sleep patterns in the mouse.Brain Inj. 2014;28(4):504-10. doi: 10.3109/02699052.2014.888768. Epub 2014 Apr 4. Brain Inj. 2014. PMID: 24702469 Free PMC article.
-
Acute Post-Traumatic Sleep May Define Vulnerability to a Second Traumatic Brain Injury in Mice.J Neurotrauma. 2019 Apr 15;36(8):1318-1334. doi: 10.1089/neu.2018.5980. Epub 2018 Dec 18. J Neurotrauma. 2019. PMID: 30398389 Free PMC article.
-
Recovery of neurological function despite immediate sleep disruption following diffuse brain injury in the mouse: clinical relevance to medically untreated concussion.Sleep. 2014 Apr 1;37(4):743-52. doi: 10.5665/sleep.3582. Sleep. 2014. PMID: 24899763 Free PMC article.
-
Traumatic brain injury and disturbed sleep and wakefulness.Neuromolecular Med. 2012 Sep;14(3):205-12. doi: 10.1007/s12017-012-8178-x. Epub 2012 Mar 23. Neuromolecular Med. 2012. PMID: 22441999 Review.
Cited by
-
Sleep, inflammation, and hemodynamics in rodent models of traumatic brain injury.Front Neurosci. 2024 Feb 15;18:1361014. doi: 10.3389/fnins.2024.1361014. eCollection 2024. Front Neurosci. 2024. PMID: 38426017 Free PMC article. Review.
-
Glial immune-related pathways mediate effects of closed head traumatic brain injury on behavior and lethality in Drosophila.PLoS Biol. 2022 Jan 26;20(1):e3001456. doi: 10.1371/journal.pbio.3001456. eCollection 2022 Jan. PLoS Biol. 2022. PMID: 35081110 Free PMC article.
-
Acute over-the-counter pharmacological intervention does not adversely affect behavioral outcome following diffuse traumatic brain injury in the mouse.Exp Brain Res. 2014 Sep;232(9):2709-19. doi: 10.1007/s00221-014-3948-3. Epub 2014 Apr 24. Exp Brain Res. 2014. PMID: 24760409
-
MW151 Inhibited IL-1β Levels after Traumatic Brain Injury with No Effect on Microglia Physiological Responses.PLoS One. 2016 Feb 12;11(2):e0149451. doi: 10.1371/journal.pone.0149451. eCollection 2016. PLoS One. 2016. PMID: 26871438 Free PMC article.
-
Dexmedetomidine-mediated sleep phase modulation ameliorates motor and cognitive performance in a chronic blast-injured mouse model.Front Neurol. 2022 Nov 1;13:1040975. doi: 10.3389/fneur.2022.1040975. eCollection 2022. Front Neurol. 2022. PMID: 36388181 Free PMC article.
References
-
- Albensi BC, Janigro D (2003) Traumatic brain injury and its effects on synaptic plasticity. Brain Inj 17: 653–663. - PubMed
-
- Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, et al. (2002) A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology 16: 514–523. - PubMed
-
- Masel BE, DeWitt DS (2010) Traumatic brain injury: a disease process, not an event. J Neurotrauma 27: 1529–1540. - PubMed
-
- Arciniegas DB, Topkoff J, Silver JM (2000) Neuropsychiatric Aspects of Traumatic Brain Injury. Curr Treat Options Neurol 2: 169–186. - PubMed
-
- Gentleman D (1999) Improving outcome after traumatic brain injury–progress and challenges. Br Med Bull 55: 910–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

