Stable expression of human muscle-specific kinase in HEp-2 M4 cells for automatic immunofluorescence diagnostics of myasthenia gravis
- PMID: 24416182
- PMCID: PMC3886972
- DOI: 10.1371/journal.pone.0083924
Stable expression of human muscle-specific kinase in HEp-2 M4 cells for automatic immunofluorescence diagnostics of myasthenia gravis
Abstract
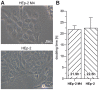
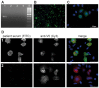
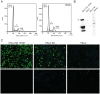
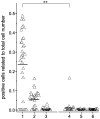
Muscle-specific kinase (MuSK) belongs to the nicotinic acetylcholine receptor complex which is targeted by pathogenic autoantibodies causing Myasthenia gravis. While up to 95% of patients with generalized Myasthenia gravis were shown to be positive for acetylcholine receptor-specific autoantibodies, up to 70% of the remaining patients develop autoantibodies against MuSK. Discrimination of the autoantibody specificity is important for therapy of Myasthenia gravis. Recently, the new automatic fluorescence assessment platform AKLIDES has been developed for immunofluorescence-based diagnostics of autoimmune diseases. In order to establish an AKLIDES procedure for the detection of MuSK-specific autoantibodies (anti-MuSK), we developed a recombinant HEp-2 cell clone expressing the human MuSK cDNA. Here we show at the mRNA and protein level that the cell clone HEp-2 M4 stably expresses human MuSK. We provide evidence for a localization of MuSK at the cell membrane. Using cell clone HEp-2 M4 on the AKLIDES system, we investigated 34 patient sera that were previously tested anti-MuSK positive by radioimmunoassay as positive controls. As negative controls, we tested 29 acetylcholine receptor-positive but MuSK-negative patient sera, 30 amytrophic lateral sclerosis (ALS) patient sera and 45 blood donors. HEp-2 M4 cells revealed a high specificity for the detection of MuSK autoantibodies from 25 patient sera assessed by a specific pattern on HEp-2 M4 cells. By using appropriate cell culture additives, the fraction of cells stained positive with anti-MuSK containing sera can be increased from 2-16% to 10-48%, depending on the serum. In conclusion, we provide data showing that the novel recombinant cell line HEp-2 M4 can be used to screen for anti-MuSK with the automatic AKLIDES system.
Conflict of interest statement
Figures
Similar articles
-
Flow Cytofluorimetric Analysis of Anti-LRP4 (LDL Receptor-Related Protein 4) Autoantibodies in Italian Patients with Myasthenia Gravis.PLoS One. 2015 Aug 18;10(8):e0135378. doi: 10.1371/journal.pone.0135378. eCollection 2015. PLoS One. 2015. PMID: 26284792 Free PMC article.
-
Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis.J Neurol. 2012 Mar;259(3):427-35. doi: 10.1007/s00415-011-6194-7. Epub 2011 Aug 5. J Neurol. 2012. PMID: 21814823
-
Anti-MuSK myasthenia gravis presenting with purely ocular findings.Arch Neurol. 2005 Jun;62(6):1002-3. doi: 10.1001/archneur.62.6.1002. Arch Neurol. 2005. PMID: 15956173
-
[Autoantibodies detected in acetylcholine receptor antibody-negative myasthenia gravis].Rinsho Byori. 2014 Mar;62(3):255-60. Rinsho Byori. 2014. PMID: 24800501 Review. Japanese.
-
Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics.Front Immunol. 2020 Feb 14;11:212. doi: 10.3389/fimmu.2020.00212. eCollection 2020. Front Immunol. 2020. PMID: 32117321 Free PMC article. Review.
Cited by
-
Clinical application of clustered-AChR for the detection of SNMG.Sci Rep. 2015 Jun 11;5:10193. doi: 10.1038/srep10193. Sci Rep. 2015. PMID: 26068604 Free PMC article.
-
Mucosal Autoimmunity to Cell-Bound GP2 Isoforms Is a Sensitive Marker in PSC and Associated With the Clinical Phenotype.Front Immunol. 2018 Aug 28;9:1959. doi: 10.3389/fimmu.2018.01959. eCollection 2018. Front Immunol. 2018. PMID: 30233574 Free PMC article. Clinical Trial.
-
Simultaneous automated screening and confirmatory testing for vasculitis-specific ANCA.PLoS One. 2014 Sep 16;9(9):e107743. doi: 10.1371/journal.pone.0107743. eCollection 2014. PLoS One. 2014. PMID: 25225805 Free PMC article.
-
Myasthenia gravis in 2025: five new things and four hopes for the future.J Neurol. 2025 Feb 22;272(3):226. doi: 10.1007/s00415-025-12922-7. J Neurol. 2025. PMID: 39987373 Free PMC article. Review.
References
-
- Phillips LH (2003) The epidemiology of myasthenia gravis. Ann N Y Acad Sci 998: 407–412. - PubMed
-
- Lang B, Vincent A (2009) Autoimmune disorders of the neuromuscular junction. Curr Opin Pharmacol 9: 336–340. - PubMed
-
- Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26: 1054–1059. - PubMed
-
- Simpson JA (1960) Myasthenia Gravis: A New Hypothesis. Scott Med J 5: 419–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

