Randomised controlled trials may underestimate drug effects: balanced placebo trial design
- PMID: 24416197
- PMCID: PMC3885519
- DOI: 10.1371/journal.pone.0084104
Randomised controlled trials may underestimate drug effects: balanced placebo trial design
Abstract
Background: It is an inherent assumption in randomised controlled trials that the drug effect can be estimated by subtracting the response during placebo from the response during active drug treatment.
Objective: To test the assumption of additivity. The primary hypothesis was that the total treatment effect is smaller than the sum of the drug effect and the placebo effect. The secondary hypothesis was that non-additivity was most pronounced in participants with large placebo effects.
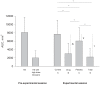
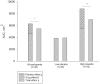
Methods: We used a within-subject randomised blinded balanced placebo design and included 48 healthy volunteers (50% males), mean (SD) age 23.4 (6.2) years. Experimental pain was induced by injections of hypertonic saline into the masseter muscle. Participants received four injections with hypertonic saline along with lidocaine or matching placebo in randomised order: A: received hypertonic saline/told hypertonic saline; B: received hypertonic saline+lidocaine/told hypertonic saline; C: received hypertonic saline+placebo/told hypertonic saline+pain killer; D: received hypertonic saline+lidocaine/told hypertonic saline+pain killer. The primary outcome measure was the area under the curve (AUC, mm(2)) of pain intensity during injections.
Results: There was a significant difference between the sum of the drug effect and the placebo effect (mean AUC 6279 mm(2) (95% CI, 4936-7622)) and the total treatment effect (mean AUC 5455 mm(2) (95% CI, 4585-6324)) (P = 0.049). This difference was larger for participants with large versus small placebo effects (P = 0.015), and the difference correlated significantly with the size of the placebo effect (r = 0.65, P = 0.006).
Conclusion: Although this study examined placebo effects and not the whole placebo response as in randomised controlled trials, it does suggest that the additivity assumption may be incorrect, and that the estimated drug effects in randomised controlled trials may be underestimated, particularly in studies reporting large placebo responses. The implications for randomised controlled trials and systematic reviews need to be discussed.
Conflict of interest statement
Figures


References
-
- Kirsch I (2000) Are drug and placebo effects in depression additive? Biol Psychiatry 47: 733–735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

