Bridging the species divide: transgenic mice humanized for type-I interferon response
- PMID: 24416207
- PMCID: PMC3887009
- DOI: 10.1371/journal.pone.0084259
Bridging the species divide: transgenic mice humanized for type-I interferon response
Abstract
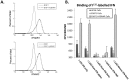
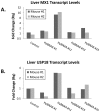
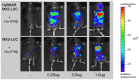
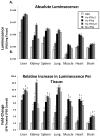
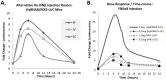
We have generated transgenic mice that harbor humanized type I interferon receptors (IFNARs) enabling the study of type I human interferons (Hu-IFN-Is) in mice. These "HyBNAR" (Hybrid IFNAR) mice encode transgenic variants of IFNAR1 and IFNAR2 with the human extracellular domains being fused to transmembrane and cytoplasmic segments of mouse sequence. B16F1 mouse melanoma cells harboring the HyBNAR construct specifically bound Hu-IFN-Is and were rendered sensitive to Hu-IFN-I stimulated anti-proliferation, STAT1 activation and activation of a prototypical IFN-I response gene (MX2). HyBNAR mice were crossed with a transgenic strain expressing the luciferase reporter gene under the control of the IFN-responsive MX2 promoter (MX2-Luciferase). Both the HyBNAR and HyBNAR/MX2-Luciferase mice were responsive to all Hu-IFN-Is tested, inclusive of IFNα2A, IFNβ, and a human superagonist termed YNSα8. The mice displayed dose-dependent pharmacodynamic responses to Hu-IFN-I injection, as assessed by measuring the expression of IFN-responsive genes. Our studies also demonstrated a weak activation of endogenous mouse interferon response, especially after high dose administration of Hu-IFNs. In sharp contrast to data published for humans, our pharmacodynamic readouts demonstrate a very short-lived IFN-I response in mice, which is not enhanced by sub-cutaneous (SC) injections in comparison to other administration routes. With algometric differences between humans and mice taken into account, the HyBNAR mice provides a convenient non-primate pre-clinical model to advance the study of human IFN-Is.
Conflict of interest statement
Figures





Similar articles
-
Enhanced in vivo efficacy of a type I interferon superagonist with extended plasma half-life in a mouse model of multiple sclerosis.J Biol Chem. 2014 Oct 17;289(42):29014-29. doi: 10.1074/jbc.M114.602474. Epub 2014 Sep 5. J Biol Chem. 2014. PMID: 25193661 Free PMC article.
-
An extracellular humanized IFNAR immunocompetent mouse model for analyses of human interferon alpha and subtypes.Emerg Microbes Infect. 2024 Dec;13(1):2287681. doi: 10.1080/22221751.2023.2287681. Epub 2024 Jan 22. Emerg Microbes Infect. 2024. PMID: 37994664 Free PMC article.
-
Validation of a HeLa Mx2/Luc reporter cell line for the quantification of human type I interferons.Pharmacology. 2009;84(3):135-44. doi: 10.1159/000235158. Epub 2009 Aug 14. Pharmacology. 2009. PMID: 19684437
-
Safety, Tolerability, and Immunogenicity of Interferons.Pharmaceuticals (Basel). 2010 Apr 20;3(4):1162-1186. doi: 10.3390/ph3041162. Pharmaceuticals (Basel). 2010. PMID: 27713294 Free PMC article. Review.
-
Identification and utility of innate immune system evasion mechanisms of ASFV.Virus Res. 2013 Apr;173(1):87-100. doi: 10.1016/j.virusres.2012.10.013. Epub 2012 Nov 16. Virus Res. 2013. PMID: 23165138 Review.
Cited by
-
Antibody-Based Targeting of Interferon-Beta-1a Mutein in HER2-Positive Cancer Enhances Antitumor Effects Through Immune Responses and Direct Cell Killing.Front Pharmacol. 2021 Jan 8;11:608774. doi: 10.3389/fphar.2020.608774. eCollection 2020. Front Pharmacol. 2021. PMID: 33505314 Free PMC article.
-
Enhanced in vivo efficacy of a type I interferon superagonist with extended plasma half-life in a mouse model of multiple sclerosis.J Biol Chem. 2014 Oct 17;289(42):29014-29. doi: 10.1074/jbc.M114.602474. Epub 2014 Sep 5. J Biol Chem. 2014. PMID: 25193661 Free PMC article.
-
Secretion of functional interferon by the type 3 secretion system of enteropathogenic Escherichia coli.Microb Cell Fact. 2024 Jun 1;23(1):163. doi: 10.1186/s12934-024-02397-y. Microb Cell Fact. 2024. PMID: 38824527 Free PMC article.
-
The Role of Type I Interferons in the Pathogenesis and Treatment of COVID-19.Front Immunol. 2020 Sep 30;11:595739. doi: 10.3389/fimmu.2020.595739. eCollection 2020. Front Immunol. 2020. PMID: 33117408 Free PMC article. Review.
-
Structure-function of type I and III interferons.Curr Opin Immunol. 2024 Feb;86:102413. doi: 10.1016/j.coi.2024.102413. Epub 2024 Apr 11. Curr Opin Immunol. 2024. PMID: 38608537 Free PMC article. Review.
References
-
- Shen X, Hong F, Nguyen VA, Gao B (2000) IL-10 attenuates IFN-alpha-activated STAT1 in the liver: involvement of SOCS2 and SOCS3. FEBS Lett 480: 132–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

