Oxidation enhances human serum albumin thermal stability and changes the routes of amyloid fibril formation
- PMID: 24416244
- PMCID: PMC3885593
- DOI: 10.1371/journal.pone.0084552
Oxidation enhances human serum albumin thermal stability and changes the routes of amyloid fibril formation
Abstract
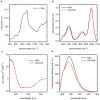
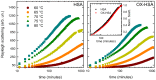
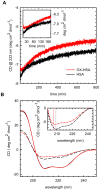
Oxidative damages are linked to several aging-related diseases and are among the chemical pathways determining protein degradation. Specifically, interplay of oxidative stress and protein aggregation is recognized to have a link to the loss of cellular function in pathologies like Alzheimer's and Parkinson's diseases. Interaction between protein and reactive oxygen species may indeed induce small changes in protein structure and lead to the inhibition/modification of protein aggregation process, potentially determining the formation of species with different inherent toxicity. Understanding the temperate relationship between these events can be of utmost importance in unraveling the molecular basis of neurodegeneration. In this work, we investigated the effect of hydrogen peroxide oxidation on Human Serum Albumin (HSA) structure, thermal stability and aggregation properties. In the selected conditions, HSA forms fibrillar aggregates, while the oxidized protein undergoes aggregation via new routes involving, in different extents, specific domains of the molecule. Minute variations due to oxidation of single residues affect HSA tertiary structure leading to protein compaction, increased thermal stability, and reduced association propensity.
Conflict of interest statement
Figures





Similar articles
-
Curcumin promotes fibril formation in F isomer of human serum albumin via amorphous aggregation.Biophys Chem. 2015 Dec;207:30-9. doi: 10.1016/j.bpc.2015.08.002. Epub 2015 Aug 15. Biophys Chem. 2015. PMID: 26298484
-
Spectroscopic analysis of the impact of oxidative stress on the structure of human serum albumin (HSA) in terms of its binding properties.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Feb 5;136 Pt B:265-82. doi: 10.1016/j.saa.2014.09.034. Epub 2014 Oct 5. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25448930
-
Resveratrol induces thermal stabilization of human serum albumin and modulates the early aggregation stage.Int J Biol Macromol. 2016 Nov;92:1049-1056. doi: 10.1016/j.ijbiomac.2016.08.014. Epub 2016 Aug 6. Int J Biol Macromol. 2016. PMID: 27506123
-
Structural, morphological, and functional diversity of amyloid oligomers.FEBS Lett. 2015 Sep 14;589(19 Pt A):2640-8. doi: 10.1016/j.febslet.2015.07.013. Epub 2015 Jul 17. FEBS Lett. 2015. PMID: 26188543 Review.
-
Exposure of Aggregation-Prone Segments is the Requirement for Amyloid Fibril Formation.Curr Protein Pept Sci. 2018;19(10):1024-1035. doi: 10.2174/1389203719666180521091647. Curr Protein Pept Sci. 2018. PMID: 29779477 Review.
Cited by
-
Oxidative Deamination of Serum Albumins by (-)-Epigallocatechin-3-O-Gallate: A Potential Mechanism for the Formation of Innate Antigens by Antioxidants.PLoS One. 2016 Apr 5;11(4):e0153002. doi: 10.1371/journal.pone.0153002. eCollection 2016. PLoS One. 2016. PMID: 27046229 Free PMC article.
-
Pathogenesis of Molar Hypomineralisation: Aged Albumin Demarcates Chalky Regions of Hypomineralised Enamel.Front Physiol. 2020 Sep 30;11:579015. doi: 10.3389/fphys.2020.579015. eCollection 2020. Front Physiol. 2020. PMID: 33101060 Free PMC article.
-
Multi-site binding of epigallocatechin gallate to human serum albumin measured by NMR and isothermal titration calorimetry.Biosci Rep. 2017 May 11;37(3):BSR20170209. doi: 10.1042/BSR20170209. Print 2017 Jun 30. Biosci Rep. 2017. PMID: 28424370 Free PMC article.
-
Applicability of FTIR-ATR Method to Measure Carbonyls in Blood Plasma after Physical and Mental Stress.Biomed Res Int. 2019 Mar 26;2019:2181370. doi: 10.1155/2019/2181370. eCollection 2019. Biomed Res Int. 2019. PMID: 31032337 Free PMC article.
-
A Comprehensive Investigation of Interactions between Antipsychotic Drug Quetiapine and Human Serum Albumin Using Multi-Spectroscopic, Biochemical, and Molecular Modeling Approaches.Molecules. 2022 Apr 18;27(8):2589. doi: 10.3390/molecules27082589. Molecules. 2022. PMID: 35458787 Free PMC article.
References
-
- Stefani M (2004) Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta 1739(1): 5–25. - PubMed
-
- Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75: 333–66. - PubMed
-
- Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational Quality Control: Folding, Refolding, and Degrading Proteins. Science 3 286: 1888–1893. - PubMed
-
- Stadtman ER (2001) Protein Oxidation in Aging and Age-Related Diseases. Annals of the New York Academy of Sciences 928: 22–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

