Matrix metalloproteinase 3 promotes cellular anti-dengue virus response via interaction with transcription factor NFκB in cell nucleus
- PMID: 24416274
- PMCID: PMC3885614
- DOI: 10.1371/journal.pone.0084748
Matrix metalloproteinase 3 promotes cellular anti-dengue virus response via interaction with transcription factor NFκB in cell nucleus
Abstract
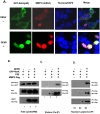
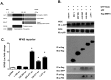
Dengue virus (DENV), the causative agent of human Dengue hemorrhagic fever, is a mosquito-borne virus of immense global health importance. Characterization of cellular factors promoting or inhibiting DENV infection is important for understanding the mechanism of DENV infection. In this report, MMP3 (stromelysin-1), a secretory endopeptidase that degrades extracellular matrices, has been shown promoting cellular antiviral response against DENV infection. Quantitative RT-PCR and Western Blot showed that the expression of MMP3 was upregulated in DENV-infected RAW264.7 cells. The intracellular viral loads were significantly higher in MMP3 silenced cells compared with controls. The expression level of selective anti-viral cytokines were decreased in MMP3 siRNA treated cells, and the transcription factor activity of NFκB was significantly impaired upon MMP3 silencing during DENV infection. Further, we found that MMP3 moved to cell nucleus upon DENV infection and colocalized with NFκB P65 in nucleus. Co-immunoprecipitation analysis suggested that MMP3 directly interacted with NFκB in nucleus during DENV infection and the C-terminal hemopexin-like domain of MMP3 was required for the interaction. This study suggested a novel role of MMP3 in nucleus during viral infection and provided new evidence for MMPs in immunomodulation.
Conflict of interest statement
Figures




References
-
- Whitehorn J, Simmons CP (2011) The pathogenesis of dengue. Vaccine 29: 7221–7228. - PubMed
-
- Murphy BR, Whitehead SS (2011) Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29: 587–619. - PubMed
-
- Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117: 1281–1283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

