New in vitro phenotypic assay for epilepsy: fluorescent measurement of synchronized neuronal calcium oscillations
- PMID: 24416277
- PMCID: PMC3885603
- DOI: 10.1371/journal.pone.0084755
New in vitro phenotypic assay for epilepsy: fluorescent measurement of synchronized neuronal calcium oscillations
Abstract
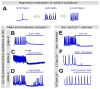
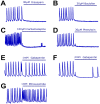
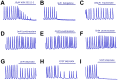
Research in the epilepsy field is moving from a primary focus on controlling seizures to addressing disease pathophysiology. This requires the adoption of resource- and time-consuming animal models of chronic epilepsy which are no longer able to sustain the testing of even moderate numbers of compounds. Therefore, new in vitro functional assays of epilepsy are needed that are able to provide a medium throughput while still preserving sufficient biological context to allow for the identification of compounds with new modes of action. Here we describe a robust and simple fluorescence-based calcium assay to measure epileptiform network activity using rat primary cortical cultures in a 96-well format. The assay measures synchronized intracellular calcium oscillations occurring in the population of primary neurons and is amenable to medium throughput screening. We have adapted this assay format to the low magnesium and the 4-aminopyridine epilepsy models and confirmed the contribution of voltage-gated ion channels and AMPA, NMDA and GABA receptors to epileptiform activity in both models. We have also evaluated its translatability using a panel of antiepileptic drugs with a variety of modes of action. Given its throughput and translatability, the calcium oscillations assay bridges the gap between simplified target-based screenings and compound testing in animal models of epilepsy. This phenotypic assay also has the potential to be used directly as a functional screen to help identify novel antiepileptic compounds with new modes of action, as well as pathways with previously unknown contribution to disease pathophysiology.
Conflict of interest statement
Figures


Similar articles
-
Measurement of intracellular Ca2+ in cultured rat embryonic hippocampal neurons using a fluorescence microplate reader: potential application to biomolecular screening.J Pharmacol Toxicol Methods. 2004 Mar-Apr;49(2):81-7. doi: 10.1016/j.vascn.2003.10.002. J Pharmacol Toxicol Methods. 2004. PMID: 14990332
-
Seizures and neurodegeneration induced by 4-aminopyridine in rat hippocampus in vivo: role of glutamate- and GABA-mediated neurotransmission and of ion channels.Neuroscience. 2000;101(3):547-61. doi: 10.1016/s0306-4522(00)00400-0. Neuroscience. 2000. PMID: 11113304
-
Research advances in basic mechanisms of seizures and antiepileptic drug action.Pharmacol Rep. 2013;65(4):787-801. doi: 10.1016/s1734-1140(13)71060-0. Pharmacol Rep. 2013. PMID: 24145073 Review.
-
Heterogeneous effects of antiepileptic drugs in an in vitro epilepsy model--a functional multineuron calcium imaging study.Eur J Neurosci. 2015 Jul;42(2):1818-29. doi: 10.1111/ejn.12945. Epub 2015 Jun 8. Eur J Neurosci. 2015. PMID: 25967117
-
[Ion channels and epilepsy].Rev Neurol. 2000 Jun;30 Suppl 1:S25-41. Rev Neurol. 2000. PMID: 10904966 Review. Spanish.
Cited by
-
Stimulation of Neurite Outgrowth in Cerebrocortical Neurons by Sodium Channel Activator Brevetoxin-2 Requires Both N-Methyl-D-aspartate Receptor 2B (GluN2B) and p21 Protein (Cdc42/Rac)-Activated Kinase 1 (PAK1).Mar Drugs. 2022 Aug 31;20(9):559. doi: 10.3390/md20090559. Mar Drugs. 2022. PMID: 36135748 Free PMC article.
-
Structure-Activity Relationship Studies in a Series of Xanthine Inhibitors of SLACK Potassium Channels.Molecules. 2024 May 22;29(11):2437. doi: 10.3390/molecules29112437. Molecules. 2024. PMID: 38893312 Free PMC article.
-
Gambierol inhibition of voltage-gated potassium channels augments spontaneous Ca2+ oscillations in cerebrocortical neurons.J Pharmacol Exp Ther. 2014 Sep;350(3):615-23. doi: 10.1124/jpet.114.215319. Epub 2014 Jun 23. J Pharmacol Exp Ther. 2014. PMID: 24957609 Free PMC article.
-
Generation and Characterization of Three Novel Mouse Mutant Strains Susceptible to Audiogenic Seizures.Cells. 2024 Oct 22;13(21):1747. doi: 10.3390/cells13211747. Cells. 2024. PMID: 39513854 Free PMC article.
-
In vitro Models for Seizure-Liability Testing Using Induced Pluripotent Stem Cells.Front Neurosci. 2018 Aug 31;12:590. doi: 10.3389/fnins.2018.00590. eCollection 2018. Front Neurosci. 2018. PMID: 30233290 Free PMC article. Review.
References
-
- Swinney DC, Anthony J (2011) How were new medicines discovered? Nat Rev Drug Discov 10: 507–519. - PubMed
-
- Eggert US (2013) The why and how of phenotypic small-molecule screens. Nat Chem Biol 9: 206–209. - PubMed
-
- Loscher W, Schmidt D (1988) Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res 2: 145–181. - PubMed
-
- Loscher W, Schmidt D (1994) Strategies in antiepileptic drug development: is rational drug design superior to random screening and structural variation? Epilepsy Res 17: 95–134. - PubMed
-
- Kupferberg H (2001) Animal models used in the screening of antiepileptic drugs. Epilepsia 42 Suppl 47–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

