Identification of an HLA-A2-restricted epitope peptide derived from hypoxia-inducible protein 2 (HIG2)
- PMID: 24416375
- PMCID: PMC3885709
- DOI: 10.1371/journal.pone.0085267
Identification of an HLA-A2-restricted epitope peptide derived from hypoxia-inducible protein 2 (HIG2)
Abstract
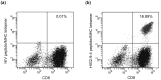
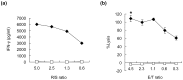
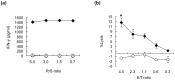
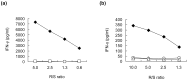
We herein report the identification of an HLA-A2 supertype-restricted epitope peptide derived from hypoxia-inducible protein 2 (HIG2), which is known to be a diagnostic marker and a potential therapeutic target for renal cell carcinoma. Among several candidate peptides predicted by the HLA-binding prediction algorithm, HIG2-9-4 peptide (VLNLYLLGV) was able to effectively induce peptide-specific cytotoxic T lymphocytes (CTLs). The established HIG2-9-4 peptide-specific CTL clone produced interferon-γ (IFN-γ) in response to HIG2-9-4 peptide-pulsed HLA-A*02:01-positive cells, as well as to cells in which HLA-A*02:01 and HIG2 were exogenously introduced. Moreover, the HIG2-9-4 peptide-specific CTL clone exerted cytotoxic activity against HIG2-expressing HLA-A*02:01-positive renal cancer cells, thus suggesting that the HIG2-9-4 peptide is naturally presented on HLA-A*02:01 of HIG-2-expressing cancer cells and is recognized by CTLs. Furthermore, we found that the HIG2-9-4 peptide could also induce CTLs under HLA-A*02:06 restriction. Taken together, these findings indicate that the HIG2-9-4 peptide is a novel HLA-A2 supertype-restricted epitope peptide that could be useful for peptide-based immunotherapy against cancer cells with HIG2 expression.
Conflict of interest statement
Figures

Similar articles
-
HER-2/neu is expressed in human renal cell carcinoma at heterogeneous levels independently of tumor grading and staging and can be recognized by HLA-A2.1-restricted cytotoxic T lymphocytes.Int J Cancer. 2000 Aug 1;87(3):349-59. Int J Cancer. 2000. PMID: 10897039
-
Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1, -A2, and -A3 alleles.J Immunol. 1998 Dec 15;161(12):6985-92. J Immunol. 1998. PMID: 9862734
-
Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes.Clin Cancer Res. 2005 Jun 15;11(12):4545-52. doi: 10.1158/1078-0432.CCR-04-2235. Clin Cancer Res. 2005. PMID: 15958640 Free PMC article.
-
Peptide FLNPDVLDI of heparanase is a novel HLA-A2-restricted CTL epitope and elicits potent immunological antitumor effects in vitro with an 8-branched design.Oncol Rep. 2013 May;29(5):1955-61. doi: 10.3892/or.2013.2347. Epub 2013 Mar 13. Oncol Rep. 2013. PMID: 23503586
-
Effective induction of cytotoxic T cells recognizing an epitope peptide derived from hypoxia-inducible protein 2 (HIG2) in patients with metastatic renal cell carcinoma.Cancer Immunol Immunother. 2017 Jan;66(1):17-24. doi: 10.1007/s00262-016-1915-5. Epub 2016 Oct 18. Cancer Immunol Immunother. 2017. PMID: 27757561 Free PMC article. Clinical Trial.
Cited by
-
Identification of a novel HLA-A24-restricted cytotoxic T lymphocyte epitope peptide derived from mesothelin in pancreatic cancer.Oncotarget. 2018 Jul 31;9(59):31448-31458. doi: 10.18632/oncotarget.25837. eCollection 2018 Jul 31. Oncotarget. 2018. PMID: 30140382 Free PMC article.
-
Effective screening of T cells recognizing neoantigens and construction of T-cell receptor-engineered T cells.Oncotarget. 2018 Jan 13;9(13):11009-11019. doi: 10.18632/oncotarget.24232. eCollection 2018 Feb 16. Oncotarget. 2018. PMID: 29541393 Free PMC article.
-
Identification of shared neoantigens derived from frameshift mutations in the APC gene.Front Immunol. 2025 May 15;16:1574955. doi: 10.3389/fimmu.2025.1574955. eCollection 2025. Front Immunol. 2025. PMID: 40443655 Free PMC article.
-
Present status and future perspective of peptide-based vaccine therapy for urological cancer.Cancer Sci. 2018 Mar;109(3):550-559. doi: 10.1111/cas.13506. Epub 2018 Feb 15. Cancer Sci. 2018. PMID: 29345737 Free PMC article. Review.
-
Identification of T Cell Receptors Targeting a Neoantigen Derived from Recurrently Mutated FGFR3.Cancers (Basel). 2023 Feb 6;15(4):1031. doi: 10.3390/cancers15041031. Cancers (Basel). 2023. PMID: 36831375 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277–300. - PubMed
-
- Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353: 2477–2490. - PubMed
-
- National Comprehensive Cancer Network (2012) NCCN Clinical Practice Guidelines in Oncology. Kidney Cancer. Version2.2012. Available: http://www.tri-kobe.org/nccn/guideline/urological/english/kidney.pdf
-
- Patil S, Ishill N, Deluca J, Motzer RJ (2010) Stage migration and increasing proportion of favorable-prognosis metastatic renal cell carcinoma patients: implications for clinical trial design and interpretation. Cancer 116: 347–354. - PubMed
-
- Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, et al. (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378: 1931–1939. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

