Mast cells play no role in the pathogenesis of postoperative ileus induced by intestinal manipulation
- PMID: 24416383
- PMCID: PMC3887017
- DOI: 10.1371/journal.pone.0085304
Mast cells play no role in the pathogenesis of postoperative ileus induced by intestinal manipulation
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/99de087d-c3fc-4e9d-9ee9-552b7524f9e1. Rodewald, Hans-Reimwer [corrected to Rodewald, Hans-Reimer]
Abstract
Introduction: Intestinal manipulation (IM) during abdominal surgery results in intestinal inflammation leading to hypomotility or ileus. Mast cell activation is thought to play a crucial role in the pathophysiology of postoperative ileus (POI). However, this conclusion was mainly drawn using mast cell-deficient mouse models with abnormal Kit signaling. These mice also lack interstitial cells of Cajal (ICC) resulting in aberrant gastrointestinal motility even prior to surgery, compromising their use as model to study POI. To avoid these experimental weaknesses we took advantage of a newly developed knock-in mouse model, Cpa3(Cre/+) , devoid of mast cells but with intact Kit signaling.
Design: The role of mast cells in the development of POI and intestinal inflammation was evaluated assessing gastrointestinal transit and muscularis externa inflammation after IM in two strains of mice lacking mast cells, i.e. Kit(W-sh/W-sh) and Cpa3(Cre/+) mice, and by use of the mast cell stabilizer cromolyn.
Results: Kit(W-sh/W-sh) mice lack ICC networks and already revealed significantly delayed gastrointestinal transit even before surgery. IM did not further delay intestinal transit, but induced infiltration of myeloperoxidase positive cells, expression of inflammatory cytokines and recruitment of monocytes and neutrophils into the muscularis externa. On the contrary, Cpa3(Cre/+) mice have a normal network of ICC and normal gastrointestinal. Surprisingly, IM in Cpa3(Cre/+) mice caused delay in gut motility and intestinal inflammation as in wild type littermates mice (Cpa3(+/+) ). Furthermore, treatment with the mast cell inhibitor cromolyn resulted in an inhibition of mast cells without preventing POI.
Conclusions: Here, we confirm that IM induced mast cell degranulation. However, our data demonstrate that mast cells are not required for the pathogenesis of POI in mice. Although there might be species differences between mouse and human, our results argue against mast cell inhibitors as a therapeutic approach to shorten POI.
Conflict of interest statement
Figures






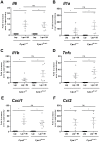
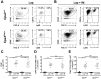
Similar articles
-
Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model.Neurogastroenterol Motil. 2012 Feb;24(2):172-84, e91. doi: 10.1111/j.1365-2982.2011.01820.x. Epub 2011 Nov 29. Neurogastroenterol Motil. 2012. PMID: 22122661
-
Effects of nerve growth factor antagonist K252a on peritoneal mast cell degranulation: implications for rat postoperative ileus.Am J Physiol Gastrointest Liver Physiol. 2015 Nov 15;309(10):G801-6. doi: 10.1152/ajpgi.00152.2015. Epub 2015 Sep 24. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26405114
-
Mast cell degranulation during abdominal surgery initiates postoperative ileus in mice.Gastroenterology. 2004 Aug;127(2):535-45. doi: 10.1053/j.gastro.2004.04.017. Gastroenterology. 2004. PMID: 15300586
-
The contribution of mast cells to postoperative ileus in experimental and clinical studies.Neurogastroenterol Motil. 2015 Jun;27(6):743-9. doi: 10.1111/nmo.12579. Neurogastroenterol Motil. 2015. PMID: 26011782 Review.
-
Intestinal mast cells in gut inflammation and motility disturbances.Biochim Biophys Acta. 2012 Jan;1822(1):66-73. doi: 10.1016/j.bbadis.2011.03.016. Epub 2011 Apr 8. Biochim Biophys Acta. 2012. PMID: 21497195 Review.
Cited by
-
Foxp3⁺ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice.PLoS Pathog. 2014 Feb 6;10(2):e1003913. doi: 10.1371/journal.ppat.1003913. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24516385 Free PMC article.
-
CO and CO-releasing molecules (CO-RMs) in acute gastrointestinal inflammation.Br J Pharmacol. 2015 Mar;172(6):1557-73. doi: 10.1111/bph.12632. Epub 2014 Jul 2. Br J Pharmacol. 2015. PMID: 24641722 Free PMC article. Review.
-
NF1+/- Hematopoietic Cells Accelerate Malignant Peripheral Nerve Sheath Tumor Development without Altering Chemotherapy Response.Cancer Res. 2017 Aug 15;77(16):4486-4497. doi: 10.1158/0008-5472.CAN-16-2643. Epub 2017 Jun 23. Cancer Res. 2017. PMID: 28646022 Free PMC article.
-
No Role for Mast Cells in Obesity-Related Metabolic Dysregulation.Front Immunol. 2016 Nov 24;7:524. doi: 10.3389/fimmu.2016.00524. eCollection 2016. Front Immunol. 2016. PMID: 27933062 Free PMC article.
-
Role of Macrophages and Mast Cells as Key Players in the Maintenance of Gastrointestinal Smooth Muscle Homeostasis and Disease.Cell Mol Gastroenterol Hepatol. 2022;13(6):1849-1862. doi: 10.1016/j.jcmgh.2022.02.017. Epub 2022 Mar 2. Cell Mol Gastroenterol Hepatol. 2022. PMID: 35245688 Free PMC article. Review.
References
-
- Boeckxstaens GE, de Jonge WJ (2009) Neuroimmune mechanisms in postoperative ileus. Gut 58: 1300–1311. - PubMed
-
- Bauer AJ, Boeckxstaens GE (2004) Mechanisms of postoperative ileus. Neurogastroenterol Motil 16 Suppl 254–60. - PubMed
-
- Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, et al. (2010) Postoperative ileus: it costs more than you expect. J Am Coll Surg 210: 228–231. - PubMed
-
- van Bree SH, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, et al. (2012) New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol (11): 675–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

