Cutaneous injury-related structural changes and their progression following topical nitrogen mustard exposure in hairless and haired mice
- PMID: 24416404
- PMCID: PMC3885697
- DOI: 10.1371/journal.pone.0085402
Cutaneous injury-related structural changes and their progression following topical nitrogen mustard exposure in hairless and haired mice
Abstract
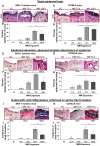
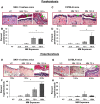
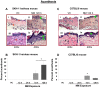
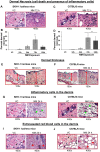
To identify effective therapies against sulfur mustard (SM)-induced skin injuries, various animals have been used to assess the cutaneous pathology and related histopathological changes of SM injuries. However, these efforts to establish relevant skin injury endpoints for efficacy studies have been limited mainly due to the restricted assess of SM. Therefore, we employed the SM analog nitrogen mustard (NM), a primary vesicating and bifunctional alkylating agent, to establish relevant endpoints for efficient efficacy studies. Our published studies show that NM (3.2 mg) exposure for 12-120 h in both the hairless SKH-1 and haired C57BL/6 mice caused clinical sequelae of toxicity similar to SM exposure in humans. The NM-induced cutaneous pathology-related structural changes were further analyzed in this study and quantified morphometrically (as percent length or area of epidermis or dermis) of skin sections in mice showing these lesions. H&E stained skin sections of both hairless and haired mice showed that NM (12-120 h) exposure caused epidermal histopathological effects such as increased epidermal thickness, epidermal-dermal separation, necrotic/dead epidermis, epidermal denuding, scab formation, parakeratosis (24-120 h), hyperkeratosis (12-120 h), and acanthosis with hyperplasia (72-120 h). Similar NM exposure in both mice caused dermal changes including necrosis, edema, increase in inflammatory cells, and red blood cell extravasation. These NM-induced cutaneous histopathological features are comparable to the reported lesions from SM exposure in humans and animal models. This study advocates the usefulness of these histopathological parameters observed due to NM exposure in screening and optimization of rescue therapies against NM and SM skin injuries.
Conflict of interest statement
Figures

Similar articles
-
Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice.Exp Toxicol Pathol. 2014 Mar;66(2-3):129-38. doi: 10.1016/j.etp.2013.11.005. Epub 2013 Dec 25. Exp Toxicol Pathol. 2014. PMID: 24373750 Free PMC article.
-
Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice.PLoS One. 2013 Jun 24;8(6):e67557. doi: 10.1371/journal.pone.0067557. Print 2013. PLoS One. 2013. PMID: 23826320 Free PMC article.
-
Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice.Toxicol Lett. 2015 Jan 5;232(1):68-78. doi: 10.1016/j.toxlet.2014.09.022. Epub 2014 Sep 30. Toxicol Lett. 2015. PMID: 25275893
-
Research models of sulfur mustard- and nitrogen mustard-induced ocular injuries and potential therapeutics.Exp Eye Res. 2022 Oct;223:109209. doi: 10.1016/j.exer.2022.109209. Epub 2022 Aug 9. Exp Eye Res. 2022. PMID: 35961426 Review.
-
Acute and chronic effects of sulfur mustard on the skin: a comprehensive review.Cutan Ocul Toxicol. 2010 Dec;29(4):269-77. doi: 10.3109/15569527.2010.511367. Epub 2010 Sep 24. Cutan Ocul Toxicol. 2010. PMID: 20868209 Review.
Cited by
-
Mitigation of nitrogen mustard mediated skin injury by a novel indomethacin bifunctional prodrug.Exp Mol Pathol. 2016 Jun;100(3):522-31. doi: 10.1016/j.yexmp.2016.05.008. Epub 2016 May 14. Exp Mol Pathol. 2016. PMID: 27189522 Free PMC article.
-
Expression of cytokines and chemokines in mouse skin treated with sulfur mustard.Toxicol Appl Pharmacol. 2018 Sep 15;355:52-59. doi: 10.1016/j.taap.2018.06.008. Epub 2018 Jun 20. Toxicol Appl Pharmacol. 2018. PMID: 29935281 Free PMC article.
-
MG53 Mitigates Nitrogen Mustard-Induced Skin Injury.Cells. 2023 Jul 23;12(14):1915. doi: 10.3390/cells12141915. Cells. 2023. PMID: 37508578 Free PMC article.
-
Preclinical safety assessment in rats after dermal exposure to acetylcarvacrol, a potential acaricide against the brown dog tick.Toxicol Rep. 2024 Nov 26;13:101834. doi: 10.1016/j.toxrep.2024.101834. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39691818 Free PMC article.
-
Nitrogen mustard exposure of murine skin induces DNA damage, oxidative stress and activation of MAPK/Akt-AP1 pathway leading to induction of inflammatory and proteolytic mediators.Toxicol Lett. 2015 Jun 15;235(3):161-71. doi: 10.1016/j.toxlet.2015.04.006. Epub 2015 Apr 16. Toxicol Lett. 2015. PMID: 25891025 Free PMC article.
References
-
- Balali-Mood M, Hefazi M (2005) The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol 19: 297–315. - PubMed
-
- Emadi SN, Aslani J, Poursaleh Z, Izadi M, Soroush M, et al. (2012) Comparison late cutaneous complications between exposure to sulfur mustard and nerve agents. Cutan Ocul Toxicol 31: 214–219. - PubMed
-
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM (2010) Mustard gas toxicity: the acute and chronic pathological effects. J Appl Toxicol 30: 627–643. - PubMed
-
- Naraghi ZS, Mansouri P, Mortazavi M (2005) A clinicopathological study on acute cutaneous lesions induced by sulfur mustard gas (yperite). Eur J Dermatol 15: 140–145. - PubMed
-
- Wormser U, Sintov A, Brodsky B, Casillas RP, Nyska A (2004) Protective effect of topical iodine containing anti-inflammatory drugs against sulfur mustard-induced skin lesions. Arch Toxicol 78: 156–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

