Nitrite reductase activity and inhibition of H₂S biogenesis by human cystathionine ß-synthase
- PMID: 24416422
- PMCID: PMC3885727
- DOI: 10.1371/journal.pone.0085544
Nitrite reductase activity and inhibition of H₂S biogenesis by human cystathionine ß-synthase
Abstract
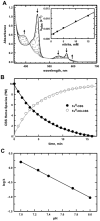
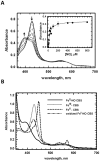
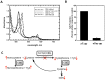
Nitrite was recognized as a potent vasodilator >130 years and has more recently emerged as an endogenous signaling molecule and modulator of gene expression. Understanding the molecular mechanisms that regulate nitrite metabolism is essential for its use as a potential diagnostic marker as well as therapeutic agent for cardiovascular diseases. In this study, we have identified human cystathionine ß-synthase (CBS) as a new player in nitrite reduction with implications for the nitrite-dependent control of H₂S production. This novel activity of CBS exploits the catalytic property of its unusual heme cofactor to reduce nitrite and generate NO. Evidence for the possible physiological relevance of this reaction is provided by the formation of ferrous-nitrosyl (Fe(II)-NO) CBS in the presence of NADPH, the human diflavin methionine synthase reductase (MSR) and nitrite. Formation of Fe(II)-NO CBS via its nitrite reductase activity inhibits CBS, providing an avenue for regulating biogenesis of H₂S and cysteine, the limiting reagent for synthesis of glutathione, a major antioxidant. Our results also suggest a possible role for CBS in intracellular NO biogenesis particularly under hypoxic conditions. The participation of a regulatory heme cofactor in CBS in nitrite reduction is unexpected and expands the repertoire of proteins that can liberate NO from the intracellular nitrite pool. Our results reveal a potential molecular mechanism for cross-talk between nitrite, NO and H₂S biology.
Conflict of interest statement
Figures

References
-
- Ignarro LJ (2000) Nitric Oxide: Biology and Pathobiology. San Diego: Academic Press.
-
- Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329: 2002–2012. - PubMed
-
- Benarroch EE (2011) Nitric oxide: A pleiotropic signal in the nervous system. Neurology 77: 1568–1576. - PubMed
-
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, et al. (2006) Free radical biology and medicine: it’s a gas, man! Am J Physiol Regul Integr Comp Physiol. 291: R491–511. - PubMed
-
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, et al. (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

