TL1A induces TCR independent IL-6 and TNF-α production and growth of PLZF⁺ leukocytes
- PMID: 24416448
- PMCID: PMC3885722
- DOI: 10.1371/journal.pone.0085793
TL1A induces TCR independent IL-6 and TNF-α production and growth of PLZF⁺ leukocytes
Abstract
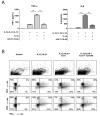
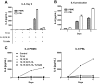
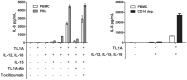
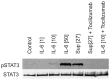
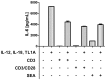
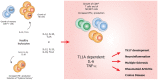
An elevated level of the cytokine TL1A is known to be associated with several autoimmune diseases, e.g. rheumatoid arthritis and inflammatory bowel disease. However, the mode of action of TL1A remains elusive. In this study, we investigated the role of TL1A in a pro-inflammatory setting, using human leukocytes purified from healthy donors. We show that TL1A, together with IL-12, IL-15 and IL-18, directly induces the production of IL-6 and TNF-α from leukocytes. Interestingly, TL1A-induced IL-6 was not produced by CD14⁺ monocytes. We further show that the produced IL-6 is fully functional, as measured by its ability to signal through the IL-6 receptor, and that the induction of IL-6 is independent of TCR stimulation. Furthermore, the transcription factor PLZF was induced in stimulated cells. These results offer a substantial explanation for the role of TL1A, since TNF-α and IL-6 are directly responsible for much of the inflammatory state in many autoimmune diseases. Our study suggests that TL1A is a possible target for the treatment of autoimmune diseases.
Conflict of interest statement
Figures



Similar articles
-
TL1A increases expression of CD25, LFA-1, CD134 and CD154, and induces IL-22 and GM-CSF production from effector CD4 T-cells.PLoS One. 2014 Aug 22;9(8):e105627. doi: 10.1371/journal.pone.0105627. eCollection 2014. PLoS One. 2014. PMID: 25148371 Free PMC article.
-
Role of TL1A in the pathogenesis of rheumatoid arthritis.J Immunol. 2009 Oct 15;183(8):5350-7. doi: 10.4049/jimmunol.0802645. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786547
-
Serum and synovial fluid levels of tumor necrosis factor-like ligand 1A and decoy receptor 3 in rheumatoid arthritis.Cytokine. 2015 Apr;72(2):185-9. doi: 10.1016/j.cyto.2014.12.026. Epub 2015 Jan 31. Cytokine. 2015. PMID: 25647275
-
The role of TL1A and DR3 in autoimmune and inflammatory diseases.Mediators Inflamm. 2013;2013:258164. doi: 10.1155/2013/258164. Epub 2013 Dec 21. Mediators Inflamm. 2013. PMID: 24453414 Free PMC article. Review.
-
The tumor necrosis factor-like cytokine 1A/death receptor 3 cytokine system in intestinal inflammation.Curr Opin Gastroenterol. 2013 Nov;29(6):597-602. doi: 10.1097/MOG.0b013e328365d3a2. Curr Opin Gastroenterol. 2013. PMID: 24100723 Review.
Cited by
-
Acute experimental changes in mood state regulate immune function in relation to central opioid neurotransmission: a model of human CNS-peripheral inflammatory interaction.Mol Psychiatry. 2016 Feb;21(2):243-51. doi: 10.1038/mp.2015.110. Epub 2015 Aug 18. Mol Psychiatry. 2016. PMID: 26283642 Free PMC article.
-
Review: Novel Insights Into Tumor Necrosis Factor Receptor, Death Receptor 3, and Progranulin Pathways in Arthritis and Bone Remodeling.Arthritis Rheumatol. 2016 Dec;68(12):2845-2856. doi: 10.1002/art.39816. Arthritis Rheumatol. 2016. PMID: 27428882 Free PMC article. Review. No abstract available.
-
First-in-human, randomized dose-escalation study of the safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of PF-06480605 in healthy subjects.Br J Clin Pharmacol. 2020 Apr;86(4):812-824. doi: 10.1111/bcp.14187. Epub 2020 Jan 28. Br J Clin Pharmacol. 2020. PMID: 31758576 Free PMC article. Clinical Trial.
-
TL1A increases expression of CD25, LFA-1, CD134 and CD154, and induces IL-22 and GM-CSF production from effector CD4 T-cells.PLoS One. 2014 Aug 22;9(8):e105627. doi: 10.1371/journal.pone.0105627. eCollection 2014. PLoS One. 2014. PMID: 25148371 Free PMC article.
-
Cytoplasmic Citrate Flux Modulates the Immune Stimulatory NKG2D Ligand MICA in Cancer Cells.Front Immunol. 2020 Aug 11;11:1968. doi: 10.3389/fimmu.2020.01968. eCollection 2020. Front Immunol. 2020. PMID: 32849657 Free PMC article.
References
-
- Migone TS, Zhang J, Luo X, Zhuang L, Chen C, et al... (2002) TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity 16: 479–492. S1074761302002832 [pii]. - PubMed
-
- Cassatella MA, Pereira-da-Silva G, Tinazzi I, Facchetti F, Scapini P, et al.. (2007) Soluble TNF-like cytokine (TL1A) production by immune complexes stimulated monocytes in rheumatoid arthritis. J Immunol 178: 7325–7333. 178/11/7325 [pii]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

