Predicting the impact of vaccination on the transmission dynamics of typhoid in South Asia: a mathematical modeling study
- PMID: 24416466
- PMCID: PMC3886927
- DOI: 10.1371/journal.pntd.0002642
Predicting the impact of vaccination on the transmission dynamics of typhoid in South Asia: a mathematical modeling study
Abstract
Background: Modeling of the transmission dynamics of typhoid allows for an evaluation of the potential direct and indirect effects of vaccination; however, relevant typhoid models rooted in data have rarely been deployed.
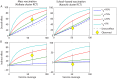
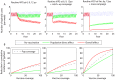
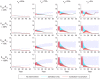
Methodology/principal findings: We developed a parsimonious age-structured model describing the natural history and immunity to typhoid infection. The model was fit to data on culture-confirmed cases of typhoid fever presenting to Christian Medical College hospital in Vellore, India from 2000-2012. The model was then used to evaluate the potential impact of school-based vaccination strategies using live oral, Vi-polysaccharide, and Vi-conjugate vaccines. The model was able to reproduce the incidence and age distribution of typhoid cases in Vellore. The basic reproductive number (R 0) of typhoid was estimated to be 2.8 in this setting. Vaccination was predicted to confer substantial indirect protection leading to a decrease in the incidence of typhoid in the short term, but (intuitively) typhoid incidence was predicted to rebound 5-15 years following a one-time campaign.
Conclusions/significance: We found that model predictions for the overall and indirect effects of vaccination depend strongly on the role of chronic carriers in transmission. Carrier transmissibility was tentatively estimated to be low, consistent with recent studies, but was identified as a pivotal area for future research. It is unlikely that typhoid can be eliminated from endemic settings through vaccination alone.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128 doi:10.1016/S0140-6736(12)61728-0 - DOI - PMC - PubMed
-
- Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, et al. (2008) A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ 86: 260–268 doi:10.2471/BLT.06.039818 - DOI - PMC - PubMed
-
- Roumagnac P, Weill F-X, Dolecek C, Baker S, Brisse S, et al. (2006) Evolutionary history of Salmonella typhi. Science (80-) 314: 1301–1304 doi:10.1126/science.1134933 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

