Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation
- PMID: 24416618
- PMCID: PMC3874981
- DOI: 10.1155/2013/684736
Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation
Abstract
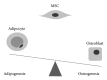
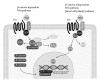
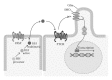
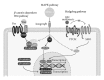
Mesenchymal stem cells (MSC) are multipotent cells, functioning as precursors to a variety of cell types including adipocytes, osteoblasts, and chondrocytes. Between osteogenic and adipogenic lineage commitment and differentiation, a theoretical inverse relationship exists, such that differentiation towards an osteoblast phenotype occurs at the expense of an adipocytic phenotype. This balance is regulated by numerous, intersecting signaling pathways that converge on the regulation of two main transcription factors: peroxisome proliferator-activated receptor- γ (PPAR γ ) and Runt-related transcription factor 2 (Runx2). These two transcription factors, PPAR γ and Runx2, are generally regarded as the master regulators of adipogenesis and osteogenesis. This review will summarize signaling pathways that govern MSC fate towards osteogenic or adipocytic differentiation. A number of signaling pathways follow the inverse balance between osteogenic and adipogenic differentiation and are generally proosteogenic/antiadipogenic stimuli. These include β -catenin dependent Wnt signaling, Hedgehog signaling, and NELL-1 signaling. However, other signaling pathways exhibit more context-dependent effects on adipogenic and osteogenic differentiation. These include bone morphogenic protein (BMP) signaling and insulin growth factor (IGF) signaling, which display both proosteogenic and proadipogenic effects. In summary, understanding those factors that govern osteogenic versus adipogenic MSC differentiation has significant implications in diverse areas of human health, from obesity to osteoporosis to regenerative medicine.
Figures

References
-
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. - PubMed
-
- Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30(5):804–810. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

