Gross Cystic Disease Fluid Protein-15(GCDFP-15)/Prolactin-Inducible Protein (PIP) as Functional Salivary Biomarker for Primary Sjögren's Syndrome
- PMID: 24416635
- PMCID: PMC3884953
- DOI: 10.4172/2157-7412.1000140
Gross Cystic Disease Fluid Protein-15(GCDFP-15)/Prolactin-Inducible Protein (PIP) as Functional Salivary Biomarker for Primary Sjögren's Syndrome
Abstract
Background: Gross cystic disease fluid protein-15(GCDFP-15)/prolactin-inducible protein (PIP) is a secretory acinar glycoprotein of 14 KDa which we have recently described as significantly lower in salivary samples of patients with primary Sjögren's syndrome (pSS) in comparison to healthy volunteers by proteomic analysis.
Aims of the study: (1) to validate our previous data on the decrease of GCDFP-15/PIP protein in a larger number of subjects with pSS (2) to integrate the proteomic results with complementary immunoassays in order better clarify the pathophysiological relevance of GCDFP-15/PIP in pSS exocrinopathy (3) to assess both the glandular expression of the GCDFP-15/PIP and the levels of glandular GCDFP-15/PIP mRNA in the patients' minor salivary gland (MSG) biopsies in order to verify whether the observed reduction of GCDFP-15/PIP in saliva may be related to a decrease in the protein production.
Patients and methods: A total of 123 salivary samples from patients affected by pSS, no-SS sicca syndrome and sex- age-matched healthy volunteers were analyzed by different proteomic techniques (SELDI-TOF-MS, 2DE, MALDI-TOF-MS). The expression of GCDFP-15/PIP was then validated by western blot analysis. Real Time PCR and immunohistochemistry for GCDFP-15/PIP in the minor salivary glands (MSG) biopsies were then carried out.
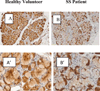
Results: By using complementary proteomic analysis we found that a putative peak of 16547 m/z was among the best independent biomarkers for pSS able to discriminate between patients and healthy controls with a sensitivity of 96 % and a specificity of 70%, with a global cross validated error of 29%. We identified the peak as the GCDFP-15/PIP protein and verified that the intensity of GCDFP-15/PIP was significantly lower in pSS patients when compared to both no-SS sicca subjects and healthy controls (p<0.0001). GCDFP-15/PIP expression also correlated with both the salivary flow rate (r=0.312, p=0.023) and MSG biopsies focus score (r=-0.377, p=0.04). Finally, immunohistochemistry confirmed that GCDFP-15/PIP staining was faint in mucus acini and Real Time PCR showed that GCDFP-15/PIP mRNA was significantly lower in pSS patients when compared to both no-SS sicca subjects and healthy controls (p=0.023) thus supporting the hypothesis that the observed reduction of GCDFP-15/PIP in pSS saliva may be related to a decrease in the protein production.
Conclusion: In this study by different complementary-omic techniques we confirmed the potential role of GCDFP-15/PIP as a novel biomarker for pSS. This finding might also be functionally important as GCDFP-15/PIP has previously been shown to bind to Aquaporin 5 (AQP5), a salivary gland water channel, critical to saliva formation that is known to be downregulated in pSS. It is likely that exploring the GCDFP-15/PIP/AQP5 axis will help better understand the mechanism of salivary gland dysfunction in pSS.
Keywords: Autoimmune disease; GCDFP-15/PIP; Sjören’s syndrome.
Figures







References
-
- Baldini C, Gallo A, Perez P, Mosca M, Alevizos I, et al. Saliva as an ideal milieu for emerging diagnostic approaches in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2012;30:785–790. - PubMed
-
- Gallo A, Baldini C, Teos L, Mosca M, Bombardieri S, et al. Emerging trends in Sjögren’s syndrome: basic and translational research. Clin Exp Rheumatol. 2012;30:779–784. - PubMed
-
- Baldini C, Giusti L, Bazzichi L, Lucacchini A, Bombardieri S. Proteomic analysis of the saliva: a clue for understanding primary from secondary Sjögren’s syndrome? Autoimmun Rev. 2008;7:185–191. - PubMed
-
- Ferraccioli G, De Santis M, Peluso G, Inzitari R, Fanali C, et al. Proteomic approaches to Sjögren’s syndrome: a clue to interpret the pathophysiology and organ involvement of the disease. Autoimmun Rev. 2010;9:622–626. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
