Redox regulation of Janus kinase: The elephant in the room
- PMID: 24416654
- PMCID: PMC3876428
- DOI: 10.4161/jkst.26141
Redox regulation of Janus kinase: The elephant in the room
Abstract
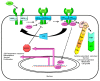
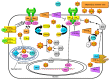
The redox regulation of Janus kinases (JAKs) is a complex subject. Due to other redox-sensitive kinases in the kinome, redox-sensitive phosphatases, and cellular antioxidant systems and reactive oxygen species (ROS) production systems, the net biological outcomes of oxidative stress on JAK-dependent signal transduction vary according to the specific biological system examined. This review begins with a discussion of the biochemical evidence for a cysteine-based redox switch in the catalytic domain of JAKs, proceeds to consider direct and indirect regulatory mechanisms involved in biological experiments, and ends with a discussion of the role(s) of redox regulation of JAKs in various diseases.
Keywords: Janus kinases; cysteine modification; cytokine biology; oxidative stress; redox regulation; thiol chemistry.
Figures

Similar articles
-
Negative regulation of Janus kinases.Cell Biochem Biophys. 2001;34(1):17-59. doi: 10.1385/CBB:34:1:17. Cell Biochem Biophys. 2001. PMID: 11394440 Review.
-
Quantitative redox proteomics: the NOxICAT method.Methods Mol Biol. 2012;893:387-403. doi: 10.1007/978-1-61779-885-6_24. Methods Mol Biol. 2012. PMID: 22665313
-
The cell cycle is a redox cycle: linking phase-specific targets to cell fate.Free Radic Biol Med. 2009 Nov 1;47(9):1282-93. doi: 10.1016/j.freeradbiomed.2009.05.026. Epub 2009 May 29. Free Radic Biol Med. 2009. PMID: 19486941 Review.
-
Redox regulation of protein kinases.FEBS J. 2013 May;280(9):1944-65. doi: 10.1111/febs.12224. Epub 2013 Mar 21. FEBS J. 2013. PMID: 23461806 Review.
-
Plant Chloroplast Stress Response: Insights from Thiol Redox Proteomics.Antioxid Redox Signal. 2020 Jul 1;33(1):35-57. doi: 10.1089/ars.2019.7823. Epub 2020 Mar 12. Antioxid Redox Signal. 2020. PMID: 31989831 Review.
Cited by
-
IL-21, Inflammatory Cytokines and Hyperpolarized CD8+ T Cells Are Central Players in Lupus Immune Pathology.Antioxidants (Basel). 2023 Jan 12;12(1):181. doi: 10.3390/antiox12010181. Antioxidants (Basel). 2023. PMID: 36671045 Free PMC article.
-
Low Density Lipoproteins Amplify Cytokine-signaling in Chronic Lymphocytic Leukemia Cells.EBioMedicine. 2017 Feb;15:24-35. doi: 10.1016/j.ebiom.2016.11.033. Epub 2016 Nov 30. EBioMedicine. 2017. PMID: 27932296 Free PMC article.
-
Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway.Int J Mol Med. 2018 Apr;41(4):1867-1876. doi: 10.3892/ijmm.2018.3419. Epub 2018 Jan 23. Int J Mol Med. 2018. PMID: 29393353 Free PMC article.
-
Redox regulation of tyrosine kinase signalling: more than meets the eye.J Biochem. 2020 Feb 1;167(2):151-163. doi: 10.1093/jb/mvz085. J Biochem. 2020. PMID: 31599960 Free PMC article. Review.
-
Targeting monoamine oxidase A: a strategy for inhibiting tumor growth with both immune checkpoint inhibitors and immune modulators.Cancer Immunol Immunother. 2024 Feb 13;73(3):48. doi: 10.1007/s00262-023-03622-0. Cancer Immunol Immunother. 2024. PMID: 38349393 Free PMC article. Review.
References
-
- Kim DS, Churchich JE. The reversible oxidation of vicinal SH groups in 4-aminobutyrate aminotransferase. Probes of conformational changes. J Biol Chem. 1987;262:14250–4. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
