JAK-STAT signaling and myocardial glucose metabolism
- PMID: 24416656
- PMCID: PMC3876426
- DOI: 10.4161/jkst.26458
JAK-STAT signaling and myocardial glucose metabolism
Abstract
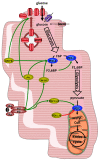
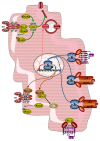
JAK-STAT signaling occurs in virtually every tissue of the body, and so does glucose metabolism. In this review, we summarize the regulation of glucose metabolism in the myocardium and ponder whether JAK-STAT signaling participates in this regulation. Despite a paucity of data directly pertaining to cardiac myocytes, we conclude that JAK-STAT signaling may contribute to the development of insulin resistance in the myocardium in response to various hormones and cytokines.
Keywords: JAK; Janus kinase; SOCS; STAT; cardiac myocytes; glucose metabolism; insulin; insulin resistance; myocardium; signal transducer and activator of transcription; suppressor of cytokine signaling.
Figures
References
-
- Opie LH. The Heart: Physiology, from Cell to Circulation 3rd ed. Philadelphia, New York: Lippincott-Raven; 1998.
-
- Stryer L. Biochemistry 2nd ed. San Francisco: W.H. Freeman and Company; 1981.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
