Plasma gelsolin protects HIV-1 gp120-induced neuronal injury via voltage-gated K+ channel Kv2.1
- PMID: 24416794
- PMCID: PMC3904794
Plasma gelsolin protects HIV-1 gp120-induced neuronal injury via voltage-gated K+ channel Kv2.1
Abstract
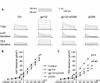
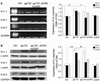
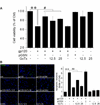
Plasma gelsolin (pGSN), a secreted form of gelsolin, is constitutively expressed throughout the central nervous system (CNS). The neurons, astrocytes and oligodendrocytes are the major sources of pGSN in the CNS. It has been shown that levels of pGSN in the cerebrospinal fluid (CSF) are decreased in several neurological conditions including HIV-1-associated neurocognitive disorders (HAND). Although there is no direct evidence that a decreased level of pGSN in CSF is causally related to the pathogenesis of neurological disorders, neural cells, if lacking pGSN, are more vulnerable to cell death. To understand how GSN levels relate to neuronal injury in HAND, we studied the effects of pGSN on HIV-1 gp120-activated outward K+ currents in primary rat cortical neuronal cultures. Incubation of rat cortical neurons with gp120 enhanced the outward K+ currents induced by voltage steps and resulted in neuronal apoptosis. Treatment with pGSN suppressed the gp120-induced increase of delayed rectifier current (IK) and reduced vulnerability to gp120-induced neuronal apoptosis. Application of Guangxitoxin-1E (GxTx), a Kv2.1 specific channel inhibitor, inhibited gp120 enhancement of IK and associated neuronal apoptosis, similar effects to pGSN. Western blot and PCR analysis revealed gp120 exposure to up-regulate Kv2.1 channel expression, which was also inhibited by treatment with pGSN. Taken together, these results indicate pGSN protects neurons by suppressing gp120 enhancement of IK through Kv2.1 channels and reduction of pGSN in HIV-1-infected brain may contribute to HIV-1-associated neuropathy.
Figures





Similar articles
-
Target of HIV-1 Envelope Glycoprotein gp120-Induced Hippocampal Neuron Damage: Role of Voltage-Gated K(+) Channel Kv2.1.Viral Immunol. 2015 Nov;28(9):495-503. doi: 10.1089/vim.2015.0020. Epub 2015 Sep 22. Viral Immunol. 2015. PMID: 26393286 Free PMC article.
-
HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents.PLoS One. 2011;6(10):e25994. doi: 10.1371/journal.pone.0025994. Epub 2011 Oct 7. PLoS One. 2011. PMID: 22016798 Free PMC article.
-
The inhibitory effect of propofol on Kv2.1 potassium channel in rat parietal cortical neurons.Neurosci Lett. 2016 Mar 11;616:93-7. doi: 10.1016/j.neulet.2016.01.058. Epub 2016 Jan 29. Neurosci Lett. 2016. PMID: 26828304
-
Voltage-gated K+ channel modulators as neuroprotective agents.Life Sci. 2010 May 22;86(21-22):775-80. doi: 10.1016/j.lfs.2010.04.004. Epub 2010 Apr 10. Life Sci. 2010. PMID: 20385147 Review.
-
Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation.Biochem Soc Trans. 2007 Nov;35(Pt 5):1064-8. doi: 10.1042/BST0351064. Biochem Soc Trans. 2007. PMID: 17956280 Review.
Cited by
-
Target of HIV-1 Envelope Glycoprotein gp120-Induced Hippocampal Neuron Damage: Role of Voltage-Gated K(+) Channel Kv2.1.Viral Immunol. 2015 Nov;28(9):495-503. doi: 10.1089/vim.2015.0020. Epub 2015 Sep 22. Viral Immunol. 2015. PMID: 26393286 Free PMC article.
-
Using Tinbergen's Four Questions as the Framework for a Neuroscience Capstone Course.J Undergrad Neurosci Educ. 2015 Oct 15;14(1):A46-55. eCollection 2015 Fall. J Undergrad Neurosci Educ. 2015. PMID: 26557795 Free PMC article.
-
Nicotinic Acetylcholine Receptors in HIV: Possible Roles During HAND and Inflammation.Cell Mol Neurobiol. 2018 Oct;38(7):1335-1348. doi: 10.1007/s10571-018-0603-8. Epub 2018 Jul 14. Cell Mol Neurobiol. 2018. PMID: 30008143 Free PMC article. Review.
-
Recombinant human plasma gelsolin suppresses persistent neuroinflammation and restores hippocampal neurogenesis in murine model of decompression sickness.J Neurophysiol. 2024 Dec 1;132(6):1877-1886. doi: 10.1152/jn.00332.2024. Epub 2024 Oct 30. J Neurophysiol. 2024. PMID: 39475490
-
SP6616 as a new Kv2.1 channel inhibitor efficiently promotes β-cell survival involving both PKC/Erk1/2 and CaM/PI3K/Akt signaling pathways.Cell Death Dis. 2016 May 5;7(5):e2216. doi: 10.1038/cddis.2016.119. Cell Death Dis. 2016. PMID: 27148689 Free PMC article.
References
-
- Anderson E, Zink W, Xiong H, Gendelman HE. HIV-1-associated dementia: a metabolic encephalopathy perpetrated by virus-infected and immune-competent mononuclear phagocytes. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S43–S54. - PubMed
-
- Azuma T, Koths K, Flanagan L, Kwiatkowski D. Gelsolin in complex with phosphatidylinositol 4,5-bisphosphate inhibits caspase-3 and −9 to retard apoptotic progression. J Biol Chem. 2000;275:3761–3766. - PubMed
-
- Bucki R, Levental I, Kulakowska A, Janmey PA. Plasma gelsolin: function, prognostic value, and potential therapeutic use. Curr Protein Pept Sci. 2008;9:541–551. - PubMed
-
- Carro E. Gelsolin as therapeutic target in Alzheimer's disease. Expert Opin Ther Targets. 2010;14:585–592. - PubMed
-
- Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem. 2000;74:2373–2379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
