Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease
- PMID: 24417819
- PMCID: PMC4002656
- DOI: 10.1053/j.gastro.2014.01.015
Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease
Abstract
Background & aims: Very early onset inflammatory bowel diseases (VEOIBD), including infant disorders, are a diverse group of diseases found in children younger than 6 years of age. They have been associated with several gene variants. Our aim was to identify the genes that cause VEOIBD.
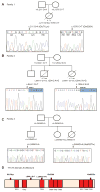
Methods: We performed whole exome sequencing of DNA from 1 infant with severe enterocolitis and her parents. Candidate gene mutations were validated in 40 pediatric patients and functional studies were carried out using intestinal samples and human intestinal cell lines.
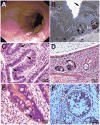
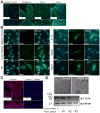
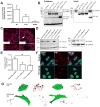
Results: We identified compound heterozygote mutations in the Tetratricopeptide repeat domain 7 (TTC7A) gene in an infant from non-consanguineous parents with severe exfoliative apoptotic enterocolitis; we also detected TTC7A mutations in 2 unrelated families, each with 2 affected siblings. TTC7A interacts with EFR3 homolog B to regulate phosphatidylinositol 4-kinase at the plasma membrane. Functional studies demonstrated that TTC7A is expressed in human enterocytes. The mutations we identified in TTC7A result in either mislocalization or reduced expression of TTC7A. Phosphatidylinositol 4-kinase was found to co-immunoprecipitate with TTC7A; the identified TTC7A mutations reduced this binding. Knockdown of TTC7A in human intestinal-like cell lines reduced their adhesion, increased apoptosis, and decreased production of phosphatidylinositol 4-phosphate.
Conclusions: In a genetic analysis, we identified loss of function mutations in TTC7A in 5 infants with VEOIBD. Functional studies demonstrated that the mutations cause defects in enterocytes and T cells that lead to severe apoptotic enterocolitis. Defects in the phosphatidylinositol 4-kinase-TTC7A-EFR3 homolog B pathway are involved in the pathogenesis of VEOIBD.
Keywords: Autoimmunity; IBD; Intestinal Atresia; Intestine.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: none
Figures


References
-
- Muise AM, Snapper SB, Kugathasan S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology. 2012;143:285–8. - PubMed
-
- Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18:509–23. - PubMed
-
- Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. - PubMed
-
- Kotlarz D, Beier R, Murugan D, et al. Loss of interleukin-10 signaling and infantile inflammatory bowel disease: implications for diagnosis and therapy. Gastroenterology. 2012;143:347–55. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R56 AI050950/AI/NIAID NIH HHS/United States
- U01 DK062413/DK/NIDDK NIH HHS/United States
- R21 DK084554/DK/NIDDK NIH HHS/United States
- P01DK046763/DK/NIDDK NIH HHS/United States
- HL59561/HL/NHLBI NIH HHS/United States
- M01 RR000425/RR/NCRR NIH HHS/United States
- MOP119457/CAPMC/ CIHR/Canada
- DK062413/DK/NIDDK NIH HHS/United States
- DK084554/DK/NIDDK NIH HHS/United States
- M01-RR00425/RR/NCRR NIH HHS/United States
- R01 DK098242/DK/NIDDK NIH HHS/United States
- R01 AI050950/AI/NIAID NIH HHS/United States
- 095688/WT_/Wellcome Trust/United Kingdom
- DK034854/DK/NIDDK NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- AI50950/AI/NIAID NIH HHS/United States
- P01 HL059561/HL/NHLBI NIH HHS/United States
- DK063491/DK/NIDDK NIH HHS/United States
- UL1 TR000124-01/TR/NCATS NIH HHS/United States
- P01 DK046763/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

