Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines
- PMID: 24418325
- PMCID: PMC3896789
- DOI: 10.1186/1756-8722-7-7
Interferon-γ-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines
Abstract
Background: Despite encouraging results with the proteasome inhibitor bortezomib in the treatment of hematologic malignancies, emergence of resistance can limit its efficacy, hence calling for novel strategies to overcome bortezomib-resistance. We previously showed that bortezomib-resistant human leukemia cell lines expressed significantly lower levels of immunoproteasome at the expense of constitutive proteasomes, which harbored point mutations in exon 2 of the PSMB5 gene encoding the β5 subunit. Here we investigated whether up-regulation of immunoproteasomes by exposure to interferon-γ restores sensitivity to bortezomib in myeloma and leukemia cell lines with acquired resistance to bortezomib.
Methods: RPMI-8226 myeloma, THP1 monocytic/macrophage and CCRF-CEM (T) parental cells and sub lines with acquired resistance to bortezomib were exposed to Interferon-γ for 24-48 h where after the effects on proteasome subunit expression and activity were measured, next to sensitivity measurements to proteasome inhibitors bortezomib, carfilzomib, and the immunoproteasome selective inhibitor ONX 0914. At last, siRNA knockdown experiments of β5i and β1i were performed to identify the contribution of these subunits to sensitivity to proteasome inhibition. Statistical significance of the differences were determined using the Mann-Whitney U test.
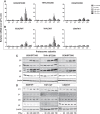
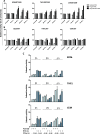
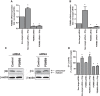
Results: Interferon-γ exposure markedly increased immunoproteasome subunit mRNA to a significantly higher level in bortezomib-resistant cells (up to 30-fold, 10-fold, and 6-fold, in β1i, β5i, and β2i, respectively) than in parental cells. These increases were paralleled by elevated immunoproteasome protein levels and catalytic activity, as well as HLA class-I. Moreover, interferon-γ exposure reinforced sensitization of bortezomib-resistant tumor cells to bortezomib and carfilzomib, but most prominently to ONX 0914, as confirmed by cell growth inhibition studies, proteasome inhibitor-induced apoptosis, activation of PARP cleavage and accumulation of polyubiquitinated proteins. This sensitization was abrogated by siRNA silencing of β5i but not by β1i silencing, prior to pulse exposure to interferon-γ.
Conclusion: Downregulation of β5i subunit expression is a major determinant in acquisition of bortezomib-resistance and enhancement of its proteasomal assembly after induction by interferon-γ facilitates restoration of sensitivity in bortezomib-resistant leukemia cells towards bortezomib and next generation (immuno) proteasome inhibitors.
Figures
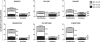


Similar articles
-
Antileukemic activity and mechanism of drug resistance to the marine Salinispora tropica proteasome inhibitor salinosporamide A (Marizomib).Mol Pharmacol. 2014 Jul;86(1):12-9. doi: 10.1124/mol.114.092114. Epub 2014 Apr 15. Mol Pharmacol. 2014. PMID: 24737138 Free PMC article.
-
Anti-leukemic activity and mechanisms underlying resistance to the novel immunoproteasome inhibitor PR-924.Biochem Pharmacol. 2014 May 1;89(1):43-51. doi: 10.1016/j.bcp.2014.02.005. Epub 2014 Feb 16. Biochem Pharmacol. 2014. PMID: 24552657
-
Higher ratio immune versus constitutive proteasome level as novel indicator of sensitivity of pediatric acute leukemia cells to proteasome inhibitors.Haematologica. 2013 Dec;98(12):1896-904. doi: 10.3324/haematol.2013.092411. Epub 2013 Sep 20. Haematologica. 2013. PMID: 24056819 Free PMC article.
-
Molecular basis of resistance to proteasome inhibitors in hematological malignancies.Drug Resist Updat. 2015 Jan;18:18-35. doi: 10.1016/j.drup.2014.12.001. Epub 2014 Dec 10. Drug Resist Updat. 2015. PMID: 25670156 Review.
-
(Immuno)proteasomes as therapeutic target in acute leukemia.Cancer Metastasis Rev. 2017 Dec;36(4):599-615. doi: 10.1007/s10555-017-9699-4. Cancer Metastasis Rev. 2017. PMID: 29071527 Free PMC article. Review.
Cited by
-
ISG15 in the tumorigenesis and treatment of cancer: An emerging role in malignancies of the digestive system.Oncotarget. 2016 Nov 8;7(45):74393-74409. doi: 10.18632/oncotarget.11911. Oncotarget. 2016. PMID: 27626310 Free PMC article. Review.
-
Antileukemic activity and mechanism of drug resistance to the marine Salinispora tropica proteasome inhibitor salinosporamide A (Marizomib).Mol Pharmacol. 2014 Jul;86(1):12-9. doi: 10.1124/mol.114.092114. Epub 2014 Apr 15. Mol Pharmacol. 2014. PMID: 24737138 Free PMC article.
-
Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration.Oncotarget. 2016 Nov 22;7(47):77543-77557. doi: 10.18632/oncotarget.12721. Oncotarget. 2016. PMID: 27769052 Free PMC article.
-
Hypoxia-induced shift in the phenotype of proteasome from 26S toward immunoproteasome triggers loss of immunoprivilege of mesenchymal stem cells.Cell Death Dis. 2020 Jun 4;11(6):419. doi: 10.1038/s41419-020-2634-6. Cell Death Dis. 2020. PMID: 32499535 Free PMC article.
-
Pre-Clinical Evaluation of the Proteasome Inhibitor Ixazomib against Bortezomib-Resistant Leukemia Cells and Primary Acute Leukemia Cells.Cells. 2021 Mar 17;10(3):665. doi: 10.3390/cells10030665. Cells. 2021. PMID: 33802801 Free PMC article.
References
-
- Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, Jiang J, Laidig GJ, Lewis ER, Parlati F, Shenk KD, Smyth MS, Sun CM, Vallone MK, Woo TM, Molineaux CJ, Bennett MK. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. - DOI - PubMed
-
- Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Trudel S, Kukreti V, Bahlis N, Alsina M, Chanan-Khan A, Buadi F, Reu FJ, Somlo G, Zonder J, Song K, Stewart AK, Stadtmauer E, Kunkel L, Wear S, Wong AF, Orlowski RZ, Jagannath S. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

