H2S and its role in redox signaling
- PMID: 24418393
- PMCID: PMC4048824
- DOI: 10.1016/j.bbapap.2014.01.002
H2S and its role in redox signaling
Abstract
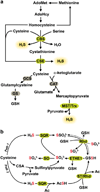
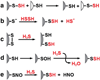
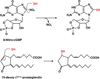
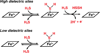
Hydrogen sulfide (H2S) has emerged as an important gaseous signaling molecule that is produced endogenously by enzymes in the sulfur metabolic network. H2S exerts its effects on multiple physiological processes important under both normal and pathological conditions. These functions include neuromodulation, regulation of blood pressure and cardiac function, inflammation, cellular energetics and apoptosis. Despite the recognition of its biological importance and its beneficial effects, the mechanism of H2S action and the regulation of its tissue levels remain unclear in part owing to its chemical and physical properties that render handling and analysis challenging. Furthermore, the multitude of potential H2S effects has made it difficult to dissect its signaling mechanism and to identify specific targets. In this review, we focus on H2S metabolism and provide an overview of the recent literature that sheds some light on its mechanism of action in cellular redox signaling in health and disease. This article is part of a Special Issue entitled: Thiol-Based Redox Processes.
Keywords: Hydrogen sulfide; Redox; Thiol.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

