Extracellular matrix: a dynamic microenvironment for stem cell niche
- PMID: 24418517
- PMCID: PMC4081568
- DOI: 10.1016/j.bbagen.2014.01.010
Extracellular matrix: a dynamic microenvironment for stem cell niche
Abstract
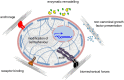
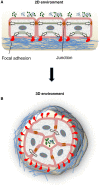
Background: Extracellular matrix (ECM) is a dynamic and complex environment characterized by biophysical, mechanical and biochemical properties specific for each tissue and able to regulate cell behavior. Stem cells have a key role in the maintenance and regeneration of tissues and they are located in a specific microenvironment, defined as niche.
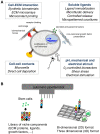
Scope of review: We overview the progresses that have been made in elucidating stem cell niches and discuss the mechanisms by which ECM affects stem cell behavior. We also summarize the current tools and experimental models for studying ECM-stem cell interactions.
Major conclusions: ECM represents an essential player in stem cell niche, since it can directly or indirectly modulate the maintenance, proliferation, self-renewal and differentiation of stem cells. Several ECM molecules play regulatory functions for different types of stem cells, and based on its molecular composition the ECM can be deposited and finely tuned for providing the most appropriate niche for stem cells in the various tissues. Engineered biomaterials able to mimic the in vivo characteristics of stem cell niche provide suitable in vitro tools for dissecting the different roles exerted by the ECM and its molecular components on stem cell behavior.
General significance: ECM is a key component of stem cell niches and is involved in various aspects of stem cell behavior, thus having a major impact on tissue homeostasis and regeneration under physiological and pathological conditions. This article is part of a Special Issue entitled Matrix-mediated cell behaviour and properties.
Keywords: Cell receptor; Extracellular matrix; Growth factor; Stem cell; Stem cell niche; Tissue engineering.
Copyright © 2014. Published by Elsevier B.V.
Figures


References
-
- Watt F.M., Huck W.T.S. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013;14:467–473. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

