Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging
- PMID: 24418545
- PMCID: PMC3902144
- DOI: 10.1016/j.virol.2013.11.017
Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging
Abstract
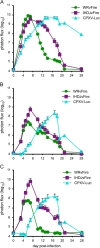
Classical inbred mice are extensively used for virus research. However, we recently found that some wild-derived inbred mouse strains are more susceptible than classical strains to monkeypox virus. Experiments described here indicated that the 50% lethal dose of vaccinia virus (VACV) and cowpox virus (CPXV) were two logs lower in wild-derived inbred CAST/Ei mice than classical inbred BALB/c mice, whereas there was little difference in the susceptibility of the mouse strains to herpes simplex virus. Live bioluminescence imaging was used to follow spread of pathogenic and attenuated VACV strains and CPXV virus from nasal passages to organs in the chest and abdomen of CAST/Ei mice. Luminescence increased first in the head and then simultaneously in the chest and abdomen in a dose-dependent manner. The spreading kinetics was more rapid with VACV than CPXV although the peak photon flux was similar. These data suggest advantages of CAST/Ei mice for orthopoxvirus studies.
Keywords: Cowpox virus pathogenesis; Poxvirus pathogenesis; Vaccinia virus pathogenesis; Wild-derived inbred mice.
© 2013 Published by Elsevier Inc.
Figures

Similar articles
-
Adaptive Immune Response to Vaccinia Virus LIVP Infection of BALB/c Mice and Protection against Lethal Reinfection with Cowpox Virus.Viruses. 2021 Aug 17;13(8):1631. doi: 10.3390/v13081631. Viruses. 2021. PMID: 34452494 Free PMC article.
-
Insufficient Innate Immunity Contributes to the Susceptibility of the Castaneous Mouse to Orthopoxvirus Infection.J Virol. 2017 Sep 12;91(19):e01042-17. doi: 10.1128/JVI.01042-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747505 Free PMC article.
-
Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses.Antimicrob Agents Chemother. 2002 May;46(5):1329-35. doi: 10.1128/AAC.46.5.1329-1335.2002. Antimicrob Agents Chemother. 2002. PMID: 11959564 Free PMC article.
-
[Research progress in the structure and fuction of Orthopoxvirus host range genes].Bing Du Xue Bao. 2013 Jun;29(4):437-41. Bing Du Xue Bao. 2013. PMID: 23895011 Review. Chinese.
-
Buffalopox.Vopr Virusol. 2016;61(5):200-4. Vopr Virusol. 2016. PMID: 29323851 Review.
Cited by
-
Longitudinal bioluminescent imaging of HIV-1 infection during antiretroviral therapy and treatment interruption in humanized mice.PLoS Pathog. 2019 Dec 5;15(12):e1008161. doi: 10.1371/journal.ppat.1008161. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31805155 Free PMC article.
-
Attenuation of monkeypox virus by deletion of genomic regions.Virology. 2015 Jan 15;475:129-38. doi: 10.1016/j.virol.2014.11.009. Epub 2014 Dec 1. Virology. 2015. PMID: 25462353 Free PMC article.
-
Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni).Virology. 2015 Jan 15;475:150-8. doi: 10.1016/j.virol.2014.11.015. Epub 2014 Dec 2. Virology. 2015. PMID: 25462355 Free PMC article.
-
Vaccinia virus and Cowpox virus are not susceptible to the interferon-induced antiviral protein MxA.PLoS One. 2017 Jul 20;12(7):e0181459. doi: 10.1371/journal.pone.0181459. eCollection 2017. PLoS One. 2017. PMID: 28727764 Free PMC article.
-
Characterization of a large, proteolytically processed cowpox virus membrane glycoprotein conserved in most chordopoxviruses.Virology. 2015 Sep;483:209-17. doi: 10.1016/j.virol.2015.04.014. Epub 2015 May 15. Virology. 2015. PMID: 25980741 Free PMC article.
References
-
- Beck J.A., Lloyd S., Hafezparast M., Lennon-Pierce M., Eppig J.T., Festing M.F., Fisher E.M. Genealogies of mouse inbred strains. Nat. Genet. 2000;24:23–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

