Sox17 is required for normal pulmonary vascular morphogenesis
- PMID: 24418654
- PMCID: PMC4422074
- DOI: 10.1016/j.ydbio.2013.11.018
Sox17 is required for normal pulmonary vascular morphogenesis
Abstract
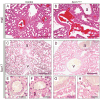
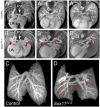
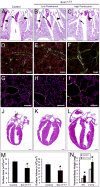
The SRY-box containing transcription factor Sox17 is required for endoderm formation and vascular morphogenesis during embryonic development. In the lung, Sox17 is expressed in mesenchymal progenitors of the embryonic pulmonary vasculature and is restricted to vascular endothelial cells in the mature lung. Conditional deletion of Sox17 in splanchnic mesenchyme-derivatives using Dermo1-Cre resulted in substantial loss of Sox17 from developing pulmonary vascular endothelial cells and caused pulmonary vascular abnormalities before birth, including pulmonary vein varices, enlarged arteries, and decreased perfusion of the microvasculature. While survival of Dermo1-Cre;Sox17Δ/Δ mice (herein termed Sox17Δ/Δ) was unaffected at E18.5, most Sox17Δ/Δ mice died by 3 weeks of age. After birth, the density of the pulmonary microvasculature was decreased in association with alveolar simplification, biventricular cardiac hypertrophy, and valvular regurgitation. The severity of the postnatal cardiac phenotype was correlated with the severity of pulmonary vasculature abnormalities. Sox17 is required for normal formation of the pulmonary vasculature and postnatal cardiovascular homeostasis.
Keywords: Dermo1-Cre; Endothelial; Lung; Sox17; Vascular morphogenesis.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Sox7, Sox17, and Sox18 Cooperatively Regulate Vascular Development in the Mouse Retina.PLoS One. 2015 Dec 2;10(12):e0143650. doi: 10.1371/journal.pone.0143650. eCollection 2015. PLoS One. 2015. PMID: 26630461 Free PMC article.
-
Sox17 is indispensable for acquisition and maintenance of arterial identity.Nat Commun. 2013;4:2609. doi: 10.1038/ncomms3609. Nat Commun. 2013. PMID: 24153254 Free PMC article.
-
Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse.Development. 2013 Aug;140(15):3128-38. doi: 10.1242/dev.088765. Epub 2013 Jul 3. Development. 2013. PMID: 23824574
-
SOX17 in cellular reprogramming and cancer.Semin Cancer Biol. 2020 Dec;67(Pt 1):65-73. doi: 10.1016/j.semcancer.2019.08.008. Epub 2019 Aug 13. Semin Cancer Biol. 2020. PMID: 31419525 Review.
-
Transcription Factors Regulating Embryonic Development of Pulmonary Vasculature.Adv Anat Embryol Cell Biol. 2018;228:1-20. doi: 10.1007/978-3-319-68483-3_1. Adv Anat Embryol Cell Biol. 2018. PMID: 29288383 Review.
Cited by
-
Genetics and Other Omics in Pediatric Pulmonary Arterial Hypertension.Chest. 2020 May;157(5):1287-1295. doi: 10.1016/j.chest.2020.01.013. Epub 2020 Jan 30. Chest. 2020. PMID: 32006592 Free PMC article. Review.
-
Loss of Fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis.JCI Insight. 2020 Dec 8;6(1):e141618. doi: 10.1172/jci.insight.141618. JCI Insight. 2020. PMID: 33290280 Free PMC article.
-
ETV2 Overexpression Promotes Efficient Differentiation of Pluripotent Stem Cells to Endothelial Cells.Biotechnol Bioeng. 2025 Jul;122(7):1914-1928. doi: 10.1002/bit.28979. Epub 2025 Mar 25. Biotechnol Bioeng. 2025. PMID: 40134129 Free PMC article.
-
Sox17 Deficiency Promotes Pulmonary Arterial Hypertension via HGF/c-Met Signaling.Circ Res. 2022 Oct 28;131(10):792-806. doi: 10.1161/CIRCRESAHA.122.320845. Epub 2022 Oct 7. Circ Res. 2022. PMID: 36205124 Free PMC article.
-
Predicting gene regulatory networks from cell atlases.Life Sci Alliance. 2020 Sep 21;3(11):e202000658. doi: 10.26508/lsa.202000658. Print 2020 Nov. Life Sci Alliance. 2020. PMID: 32958603 Free PMC article.
References
-
- Ahnfelt-Ronne J, Jorgensen MC, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An improved method for three-dimensional reconstruction of protein expression patterns in intact mouse and chicken embryos and organs. J Histochem Cytochem. 2007;55:925–930. - PubMed
-
- Ben-menachem Y, Kuroda K, Kyger ER, Brest AN, Copeland OP, Coan JD. The various forms of pulmonary varices. report of three cases and review of the literature. Am J Roentgenol Radium Ther Nucl Med. 1975;125:881–889. - PubMed
-
- Brachtendorf G, Kuhn A, Samulowitz U, Knorr R, Gustafsson E, Potocnik AJ, Fassler R, Vestweber D. Early expression of endomucin on endothelium of the mouse embryo and on putative hematopoietic clusters in the dorsal aorta. Dev Dyn. 2001;222:410–419. - PubMed
-
- Burtscher I, Barkey W, Schwarzfischer M, Theis FJ, Lickert H. The Sox17-mCherry fusion mouse line allows visualization of endoderm and vascular endothelial development. Genesis. 2012;50:496–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

