Metakaryotic stem cell nuclei use pangenomic dsRNA/DNA intermediates in genome replication and segregation
- PMID: 24418910
- PMCID: PMC4049894
- DOI: 10.4161/org.27684
Metakaryotic stem cell nuclei use pangenomic dsRNA/DNA intermediates in genome replication and segregation
Abstract
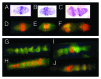
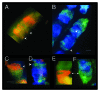
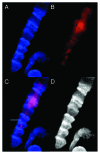
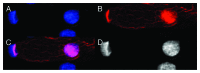
Bell shaped nuclei of metakaryotic cells double their DNA content during and after symmetric and asymmetric amitotic fissions rather than in the separate, pre-mitotic S-phase of eukaryotic cells. A parsimonious hypothesis was tested that the two anti-parallel strands of each chromatid DNA helix were first segregated as ssDNA-containing complexes into sister nuclei then copied to recreate a dsDNA genome. Metakaryotic nuclei that were treated during amitosis with RNase A and stained with acridine orange or fluorescent antibody to ssDNA revealed large amounts of ssDNA. Without RNase treatment metakaryotic nuclei in amitosis stained strongly with an antibody complex specific to dsRNA/DNA. Images of amitotic figures co-stained with dsRNA/DNA antibody and DAPI indicated that the entire interphase dsDNA genome (B-form helices) was transformed into two dsRNA/DNA genomes (A-form helices) that were segregated in the daughter cell nuclei then retransformed into dsDNA. As this process segregates DNA strands of opposite polarity in sister cells it hypothetically offers a sequential switching mechanism within the diverging stem cell lineages of development.
Keywords: amitosis; development; differentiation; dsRNA/DNA; genome replication; metakaryotic; stem cells.
Figures

Similar articles
-
Human fetal/tumor metakaryotic stem cells: pangenomic homologous pairing and telomeric end-joining of chromatids.Cancer Genet Cytogenet. 2010 Dec;203(2):203-8. doi: 10.1016/j.cancergencyto.2010.08.015. Cancer Genet Cytogenet. 2010. PMID: 21156234 Free PMC article.
-
Metakaryotic stem cell lineages in organogenesis of humans and other metazoans.Organogenesis. 2009 Oct;5(4):191-200. doi: 10.4161/org.5.4.9632. Organogenesis. 2009. PMID: 20539738 Free PMC article.
-
Stem cell identity and template DNA strand segregation.Curr Opin Cell Biol. 2008 Dec;20(6):716-22. doi: 10.1016/j.ceb.2008.10.004. Epub 2008 Nov 25. Curr Opin Cell Biol. 2008. PMID: 18996191 Review.
-
Mutator/Hypermutable fetal/juvenile metakaryotic stem cells and human colorectal carcinogenesis.Front Oncol. 2013 Oct 29;3:267. doi: 10.3389/fonc.2013.00267. Front Oncol. 2013. PMID: 24195059 Free PMC article. Review.
-
Molecular cloaking of H2A.Z on mortal DNA chromosomes during nonrandom segregation.Stem Cells. 2011 Oct;29(10):1620-7. doi: 10.1002/stem.707. Stem Cells. 2011. PMID: 21905168
Cited by
-
Split hand/foot malformation genetics supports the chromosome 7 copy segregation mechanism for human limb development.Philos Trans R Soc Lond B Biol Sci. 2016 Dec 19;371(1710):20150415. doi: 10.1098/rstb.2015.0415. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27821526 Free PMC article. Review.
-
Arterial calcification: Finger-pointing at resident and circulating stem cells.World J Stem Cells. 2014 Nov 26;6(5):540-51. doi: 10.4252/wjsc.v6.i5.540. World J Stem Cells. 2014. PMID: 25426251 Free PMC article. Review.
-
The study of calcified atherosclerotic arteries: an alternative to evaluate the composition of a problematic tissue reveals new insight including metakaryotic cells.BMC Clin Pathol. 2016 Jul 29;16:12. doi: 10.1186/s12907-016-0036-6. eCollection 2016. BMC Clin Pathol. 2016. PMID: 27478409 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
