Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group
- PMID: 24419114
- PMCID: PMC3918536
- DOI: 10.1200/JCO.2013.51.2038
Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group
Abstract
Purpose: Platinum-based therapy is the mainstay for management of high-risk neuroblastoma. Prevalence of platinum-related ototoxicity has ranged from 13% to 95% in previous reports; variability is attributable to small samples and disparate grading scales. There is no consensus regarding optimal ototoxicity grading. Furthermore, prevalence and predictors of hearing loss in a large uniformly treated high-risk neuroblastoma population are unknown. We address these gaps in our study.
Patients and methods: Audiologic testing was completed after administration of cisplatin alone (< 400 mg/m(2); exposure one) or after cisplatin (400 mg/m(2)) plus carboplatin (1,700 mg/m(2); exposure two). Hearing loss was graded using four scales (American Speech-Language-Hearing Association; Brock; Chang; and Common Terminology Criteria for Adverse Events, version 3 [CTCAEv3]).
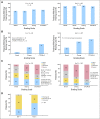
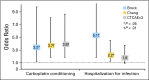
Results: Of 489 eligible patients, 333 had evaluable audiologic data. Median age at diagnosis was 3.3 years. Prevalence of severe hearing loss differed by scale. For those in the exposure-one group, prevalence ranged from 8% per Brock to 47% per CTCAEv3 (Brock v CTCAEv3 and Chang, P < .01; CTCAEv3 v Chang, P = .16); for those in the exposure-two group, prevalence ranged from 30% per Brock to 71% per CTCAEv3 (all pair-wise comparisons, P < .01). In patients requiring hearing aids, hearing loss was graded as severe in 49% (Brock), 91% (Chang), and 100% (CTCAEv3). Risk factors for severe hearing loss included exposure to cisplatin and carboplatin compared with cisplatin alone and hospitalization for infection.
Conclusion: Severe hearing loss is prevalent among children with high-risk neuroblastoma. Exposure to cisplatin combined with myeloablative carboplatin significantly increases risk. The Brock scale underestimates severe hearing loss and should be used with caution in this setting.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Brodeur GM, Hogarty MD, Mosse YP, et al. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Wolters Kluwer-Lippincott Williams and Wilkins; 2011. pp. 886–922.
-
- Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. - PubMed
-
- Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

