A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER)
- PMID: 24419411
- PMCID: PMC4132036
- DOI: 10.1097/JTO.0000000000000033
A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER)
Abstract
Introduction: PIONEER (NCT01185314) was a prospective, multinational, epidemiological study of epidermal growth factor receptor (EGFR) mutations in patients from Asia with newly diagnosed advanced lung adenocarcinoma.
Methods: Eligible patients (aged ≥20 years) had untreated stage IIIB/IV adenocarcinoma. The EGFR mutation status (primary end point: positive, negative, or undetermined) of tumor samples (biopsy, surgical specimen, or cytology) was determined (Scorpion amplification refractory mutation system). EGFR mutation frequency was calculated and compared between demographic and clinical subgroups.
Results: Of 1482 patients from seven Asian regions, 43.4% of patients were female, median age was 60 years (range, 17-94), and 52.6% of patients were never-smokers. EGFR mutation status was evaluable in tumors from 1450 patients (97.8%) (746 [51.4%] positive; 704 [48.6%] negative). Country, sex, ethnicity, smoking status, pack-years (all p < 0.001), disease stage (p = 0.009), and histology type (p = 0.016) correlated significantly with EGFR mutation frequency. Mutation frequency was 61.1% in females, 44.0% in males; lower in patients from India (22.2%) compared with other areas (47.2%-64.2%); highest among never-smokers (60.7%); and decreased as pack-year number increased (>0-10 pack-years, 57.9%; >50 pack-years, 31.4%) (similar trend by sex). Ethnic group (p < 0.001) and pack-years (p < 0.001) had statistically significant associations with mutation frequency (multivariate analysis); sex was not significant when adjusted for smoking status.
Conclusion: PIONEER is the first prospective study to confirm high EGFR mutation frequency (51.4% overall) in tumors from Asian patients with adenocarcinoma. The observed high mutation frequency in demographic/clinical subgroups compared with white populations suggests that mutation testing should be considered for all patients with stage IIIB/IV adenocarcinoma, even males and regular smokers, among Asian populations.
Conflict of interest statement
Disclosure: Drs. Heeroma and Itoh are employees of AstraZeneca and hold shares in AstraZeneca. Drs. Shi, Au, Thongprasert, Srinivasan, Khoa, and Yang do not have any conflicts of interest to disclose. Dr. Tsai has received honoraria for speech from AstraZeneca, Pfizer, Roche, Eli Lilly, and Boehringer Ingelheim. Dr. Cornelio has received consultancy fees from AstraZeneca.
Figures
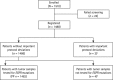
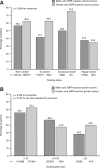
Comment in
-
To the editor.J Thorac Oncol. 2014 Jul;9(7):e57-e58. doi: 10.1097/JTO.0000000000000192. J Thorac Oncol. 2014. PMID: 24926558 No abstract available.
-
In response.J Thorac Oncol. 2014 Jul;9(7):e58-e59. doi: 10.1097/JTO.0000000000000237. J Thorac Oncol. 2014. PMID: 24926559 No abstract available.
-
EGFR mutations in Asian patients with advanced lung adenocarcinoma.J Thorac Oncol. 2014 Sep;9(9):e70-1. doi: 10.1097/JTO.0000000000000251. J Thorac Oncol. 2014. PMID: 25122441 No abstract available.
References
-
- D’Addario G, Früh M, Reck M, Baumann P, Klepetko W, Felip E ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. - PubMed
-
- Crinò L, Weder W, van Meerbeeck J, Felip E ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v103–v115. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
-
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–1128. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

