Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q
- PMID: 24419415
- PMCID: PMC4132025
- DOI: 10.1097/JTO.0000000000000048
Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q
Abstract
Introduction: In non-small-cell lung cancer, an exon 19 deletion and an L858R point mutation in the epidermal growth factor receptor (EGFR) are predictors of a response to EGFR-tyrosine kinase inhibitors. However, it is uncertain whether other uncommon EGFR mutations are associated with sensitivity to EGFR-tyrosine kinase inhibitors.
Methods: A post-hoc analysis to assess prognostic factors was performed with the use of patients with EGFR mutations (exon 19 deletion, L858R, G719X, and L861Q) who were treated with gefitinib in the NEJ002 study, which compared gefitinib with carboplatin-paclitaxel as the first-line therapy.
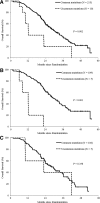
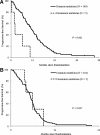
Results: In the NEJ002 study, 225 patients with EGFR mutations received gefitinib at any treatment line. The Cox proportional hazards model indicated that performance status, response to chemotherapy, response to gefitinib, and mutation types were significant prognostic factors. Overall survival (OS) was significantly shorter among patients with uncommon EGFR mutations (G719X or L861Q) compared with OS of those with common EGFR mutations (12 versus 28.4 months; p = 0.002). In the gefitinib group (n = 114), patients with uncommon EGFR mutations had a significantly shorter OS (11.9 versus 29.3 months; p < 0.001). By contrast, OS was similar between patients with uncommon mutations and those with common mutations in the carboplatin-paclitaxel group (n = 111; 22.8 versus 28 months; p = 0.358).
Conclusions: The post-hoc analyses clearly demonstrated shorter survival for gefitinib-treated patients with uncommon EGFR mutations compared with the survival of those with common mutations and suggest that the first-line chemotherapy may be relatively effective for non-small-cell lung cancer with uncommon EGFR mutations.
Conflict of interest statement
Disclosure: Dr. Yoshizawa received grants and lecture fees from AstraZeneca; Dr. Maemondo received lecture fees from AstraZeneca and Chugai; Dr. Inoue received lecture fees from AstraZeneca and Chugai; Dr. Gemma received grants and lecture fees from AstraZeneca; Dr. Hagiwara received patent fees from Mitsubishi Chemical Medience, consulting fees, and lecture fees from AstraZeneca; Dr. Kobayashi received grants from Novartis, Nihon Kayaku, Chugai, Shionogi, Kyowa Kirin, Yakult, Taiho, and AstraZeneca and lecture fees from AstraZeneca, Chugai, and Bristol-Myers Squibb. The remaining authors declare no conflict of interest.
Figures
References
-
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. - PubMed
-
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. - PubMed
-
- Paez JG. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

