Structures of adenosine kinase from Trypanosoma brucei brucei
- PMID: 24419613
- PMCID: PMC3943091
- DOI: 10.1107/S2053230X13033621
Structures of adenosine kinase from Trypanosoma brucei brucei
Abstract
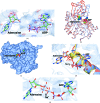
Trypanosoma brucei is a single-cellular parasite of the genus Kinetoplastida and is the causative agent of African sleeping sickness in humans. Adenosine kinase is a key enzyme in the purine-salvage pathway, phosphorylating adenosine to AMP, and also activates cytotoxic analogues such as cordycepin and Ara-A by their phosphorylation. The structures of T. brucei brucei adenosine kinase (TbAK) in its unliganded open conformation and complexed with adenosine and ADP in the closed conformation are both reported to 2.6 Å resolution. The structures give insight into the binding mode of the substrates and the conformational change induced upon substrate binding. This information can be used to guide the improvement of cytotoxic substrate analogues as potential antitrypanosomal drugs.
Keywords: Trypanosoma brucei brucei; adenosine kinase; ligand complex.
Figures

Similar articles
-
Adenosine kinase mediates high affinity adenosine salvage in Trypanosoma brucei.J Biol Chem. 2008 Feb 29;283(9):5380-8. doi: 10.1074/jbc.M705603200. Epub 2007 Dec 31. J Biol Chem. 2008. PMID: 18167353
-
Adenosine kinase of Trypanosoma brucei and its role in susceptibility to adenosine antimetabolites.Antimicrob Agents Chemother. 2007 Nov;51(11):3895-901. doi: 10.1128/AAC.00458-07. Epub 2007 Aug 13. Antimicrob Agents Chemother. 2007. PMID: 17698621 Free PMC article.
-
A bisubstrate analog induces unexpected conformational changes in phosphoglycerate kinase from Trypanosoma brucei.J Mol Biol. 1998 Jun 26;279(5):1137-48. doi: 10.1006/jmbi.1998.1835. J Mol Biol. 1998. PMID: 9642090
-
Adenosine analogues as inhibitors of Trypanosoma brucei phosphoglycerate kinase: elucidation of a novel binding mode for a 2-amino-N(6)-substituted adenosine.J Med Chem. 2000 Nov 2;43(22):4135-50. doi: 10.1021/jm000287a. J Med Chem. 2000. PMID: 11063610
-
Targeting the nucleotide metabolism of Trypanosoma brucei and other trypanosomatids.FEMS Microbiol Rev. 2023 May 19;47(3):fuad020. doi: 10.1093/femsre/fuad020. FEMS Microbiol Rev. 2023. PMID: 37156497 Free PMC article. Review.
Cited by
-
A monomer-dimer switch modulates the activity of plant adenosine kinase.J Exp Bot. 2025 Aug 21;76(12):3457-3479. doi: 10.1093/jxb/eraf094. J Exp Bot. 2025. PMID: 40063605 Free PMC article.
-
Causes and Effects of Loss of Classical Nonhomologous End Joining Pathway in Parasitic Eukaryotes.mBio. 2019 Jul 16;10(4):e01541-19. doi: 10.1128/mBio.01541-19. mBio. 2019. PMID: 31311886 Free PMC article.
-
Differentially Expressed Homologous Genes Reveal Interspecies Differences of Paragonimus Proliferus based on Transcriptome Analysis.Helminthologia. 2020 Aug 5;57(3):196-210. doi: 10.2478/helm-2020-0029. eCollection 2020 Sep. Helminthologia. 2020. PMID: 32855607 Free PMC article.
-
The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases-Part III: In-Silico Molecular Docking Investigations.Molecules. 2016 Oct 19;21(10):1389. doi: 10.3390/molecules21101389. Molecules. 2016. PMID: 27775577 Free PMC article. Review.
-
Adenosine Kinase couples sensing of cellular potassium depletion to purine metabolism.Sci Rep. 2018 Aug 10;8(1):11988. doi: 10.1038/s41598-018-30418-5. Sci Rep. 2018. PMID: 30097648 Free PMC article.
References
-
- Arvai, A. (2012). adxv: A Program to Display X-ray Diffraction Images http://www.scripps.edu/~arvai/adxv.html.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

